Building a Better Cornea
Collagen cross-linking and transepithelial PRK, used together, strengthen and reshape the cornea in this innovative treatment for post-LASIK ectasia.
By Anastasios John Kanellopoulos, MD
Progressive, asymmetrical corneal steepening associated with an increase in myopic and astigmatic refractive errors, combined with mid-peripheral and/or peripheral corneal thinning, represents a constellation of findings in ectatic corneal disorders, such as keratoconus and pellucid marginal degeneration. Asymmetry in presentation and unpredictability of progression associated with myriad topographic abnormal findings describe these entities.
Similar findings following LASIK have been described as post-LASIK ectasia.1,3 Analysis of different series of eyes developing post-LASIK ectasia has suggested that certain preoperative and/or operative features may be associated with this adverse outcome of LASIK or PRK surgery.4 The fact that ectasia can occur in the absence of these features, or that it does not occur in spite of the presence of them,5 has confounded our understanding of this complicated entity. Nevertheless, post-LASIK ectasia is a visually-disabling complication whose ultimate surgical treatment is penetrating keratoplasty when glasses or contact lenses can no longer provide patients with the vision quality to permit their activities of daily living.
Fortunately, recent advances have yielded less invasive solutions. Over the last 10 years, the use of topical riboflavin-A combined with ultraviolet-A irradiation to increase collagen cross-linking (CXL) has demonstrated the potential for retarding or eliminating the progression of keratoconus and post-LASIK ectasia. Once the progression has stabilized, it is possible to treat the surface of the eye with customized photorefractive keratectomy to normalize the corneal surface by reducing irregular astigmatism and potentially reducing the refractive error as well, providing improved visual outcomes in addition to the stabilization of the disease process.6-8
The Athens Protocol
We have successfully introduced these two intervention modalities, combined on the same day, to manage keratoconus.9,10 We refer to this approach as the Athens protocol and describe it below as a remedy for post-LASIK ectasia.
• Diagnosis of ectasia. Patients who developed progressive corneal steepening associated with an increasing myopic and/or astigmatic refractive errors two or more months following LASIK surgery were diagnosed with ectasia. These findings were combined with increasing inferior corneal steepening and thinning based on videokeratography and ultrasound pachymetry. The pre-LASIK operative clinical data and topography were requested from the referring physicians or primary LASIK surgeons for analysis. Progression of the myopic refractive error with or without progression of the manifest astigmatism, decreasing UCVA, loss of BSCVA, progressive inferior corneal steepening on topography and/or decreasing inferior corneal thickness were findings in all of our cases.
Each patient underwent a manifest refraction, as well as measurement of the uncorrected distance visual acuity (UDVA or UCVA) and best spectacle-corrected visual acuity (CDVA or BSCVA) by Snellen acuity testing. Cycloplegic refractions were performed with 1% tropicamide (Alcon Laboratories). A slit-lamp exam confirmed the presence of a LASIK flap. Keratometry readings were obtained by videokeratography (Topolyzer, Wavelight) and/or a manual keratometer (Bausch + Lomb). Pachymetry was performed using at least one of the following devices: the Pentacam (Oculus), the Orbscan II (Bausch + Lomb) or the EchoScan US-1800 (Nidek). Specular microscopy was performed using the Konan specular microscope (Konan Medical). Topography was performed using the Orbscan II or the Pentacam (Oculus) systems.
• Surgical step 1: The (partial, spherically corrected) topography-guided transepithelial PRK technique. We devised this technique based on the proprietary Wavelight customized platform, which can be used to normalize irregular corneas, and even ectasias. This customized excimer laser treatment is guided by topography images, and is completely different than wavefront-guided treatments. Although it has received CE mark for clinical use in the European Union since 2003, it has not yet received FDA approval.
This software uses topographic data from the Topolyzer, a placido-disc device (Wavelight). By default, it permits the consideration of eight topographies (of pre-determined threshold accuracy), averages the data and enables the surgeon to adjust the desired post-operative corneal asphericity (chosen as zero in all cases), and permits the inclusion, or not, of tilt correction (no tilt was chosen in all cases), as well the adjustment of sphere, cylinder, axis and treatment zone (OZ of 5.5 mm was chosen in all cases). The image of the planned surgery is generated by the laser software (Figure 3c).
We used partial topography-guided PRK to normalize the cornea, by reducing irregular astigmatism while treating part of the refractive error. To remove the minimum possible tissue, we decreased the effective optical zone diameter to 5.5 mm in all cases (compared to our usual treatment diameter in routine PRK and LASIK cases of at least 6.5 mm). We also planned ~70% treatment of cylinder and sphere (up to 70%), in order not to exceed 50 microns in planned stromal removal. We chose 50 microns as the maximum ablation depth, based on our long experience with this platform treating irregular corneas.7-10
Following the placement of an aspirating lid speculum (Rumex) a 6.5-mm, 50-micron PTK was performed to remove the corneal epithelium, then the topography-guided partial PRK laser treatment was applied. A cellulose sponge soaked in mitomycin-C 0.02% solution was applied over the ablated tissue for 20 seconds, followed by irrigation with 10 ml of chilled balanced salt solution.
• Surgical step 2: Collagen cross-linking procedure. For the next 10 minutes, the 0.1% riboflavin sodium phosphate ophthalmic solution (Priavision) was applied topically every two minutes. The solution appeared to “soak” into the corneal stroma rapidly, as it was centrally devoid of Bowman's membrane. Following the initial riboflavin administration, ultraviolet light of mean 370 nm wavelength (365 to 375 nm) and 7mW/cm2 radiance at 2.5 cm was projected onto the surface of the cornea for 15 minutes (using a Keracure prototype device from Priavision), after which a bandage contact lens was inserted. The Keracure device has a built-in beeper that alerts clinicians every two minutes during the 15-minute treatment to instill the riboflavin solution in a timely fashion. A bandage contact lens was placed on the cornea at the completion of the two combined procedures.
Postoperatively, topical ofloxacin (Allergan) was used QID for the first 10 days and prednisolone acetate 1% (Pred Forte, Allergan) was used QID for 60 days. Protection from all natural light with sunglasses was encouraged, along with oral 1000 mg of vitamin C daily for 60 days postop. The bandage contact lens was removed at approximately day five following complete re-epithelialization.
All cases were evaluated before and following both treatments for UDVA (UCVA), CDVA (BSCVA), refraction, keratometry (K), tomography, pachymetry, endothelial cell counts (ECC), cornea haze on a scale 0-4: (0 = clear cornea; 1 = mild haze; 2 = moderate haze; 3 = severe haze and 4 = reticular haze, obstructing iris anatomy), and ectasia as defined by stability in mean keratometry and tomography.
Summary of Results
A total of 32 eyes with ectasia were treated in our series, occurring one to four years after LASIK. Full pre-LASIK data were only available in five cases; among those, no irregularity on topographies or tomographies was noted and no other factor of the LASIK procedure was evaluated to be high risk (eg, thick flap, residual stromal bed <250 µm). All cases had documented progression of inferior steepening in topography and/or tomography maps. Age ranged from 23 to 66 years (average 32), with gender equally divided between women and men.
The mean preoperative sphere was −7.50 D and the mean preoperative astigmatism was −2.40 D in the 32 eyes. The mean preoperative central corneal thickness was ≥525 µm in 25 of the 32 eyes. The original LASIK laser resection data was unavailable in 27 eyes, and flap thickness was assumed or calculated in the corneal OCT (Optovue). The residual stromal thickness was an average of 285 µm (210 to 355). Of all 32 ectasia cases, 16 were thought to have resulted from thicker than planned flaps (residual stromal bed averaged 230 µm, and 10 showed signs of corneal irregularity on pre-LASIK topographies, and seven had no recognizable risk factor to develop ectasia).
All PRK procedures were planned to reduce corneal thickness by a maximum of 50 microns, despite the existing refractive error, to avoid exacerbation of the ectasia. Most patients (19 patients, 25 eyes) complained of significant pain the first postoperative night, whereas others reported minimal discomfort. The mean follow up after the tgPRK/CXL was 27 months.
The UDVA (UCVA) improved in 27 eyes, was unchanged in four eyes and worsened in one; it was greater than 20/30 (+0.18 LogMAR) in 11 of 32 eyes and worse than 20/60 (+0.47 LogMAR) in two. The latest CDVA (BSCVA) was 20/40 (+0.30 LogMAR) or better in 27 of the 32 eyes and 20/25 (+0.10 LogMAR) or better in 14 eyes. The mean refractive error was decreased by more than 2.5 D in 27 of 32 eyes and appeared to increase by 0.75 in three eyes. The final spherical equivalent was −1.75 D, showing that we did not target emmetropia in this treatment, but instead the reduction of cornea irregularity.
Detailed reports on four representative cases are presented in the accompanying sidebar found below and described in the figures.
Discussion
The tgPRK treatment flattens some of the cone apex (in a fashion similar to an eccentric partial myopic PRK) but simultaneously flattens an arcuate, broader area of the cornea diametrically opposed from the cone, usually in the superior-nasal periphery; this second part of the topography-guided ablation pattern (Figure 3c) resembles part of a hyperopic treatment and as thus will cause steepening of the cornea central to it, or elevation adjacent to the cone, effectively normalizing the cornea.
We have introduced this concept as an effective tissue-sparing ablation pattern in highly irregular corneas such as ectasia occurring in keratoconus.11 It is this core concept that makes our procedure more therapeutic than refractive. We have reported7-10 that, in theory, the new “flatter” and less irregular corneal shape may perform better biomechanically in eyes with ectasia. Specifically, as the corneal apex becomes a flatter and “broader” cone (compare Figures 3A and 3B), this may redistribute the biomechanical strain from the IOP and other external to the corneal factors (such as eye-rubbing, blinking, etc.). This effect may be further enhanced with additional CXL strengthening.
The same-day simultaneous tgPRK/CXL has several advantages:
(1) The combination reduces the patient's time away from work.
(2) Performing both procedures at the same time, with the tgPRK first, appeared to minimize the potential superficial stromal scarring resulting from tgPRK (unpublished observations).
(3) When one performs tgPRK following CXL, some of the cross-linked anterior cornea is removed, minimizing the potential benefit of cross-linking (unpublished observations). We believe it may be counterintuitive to remove the cross-linked tissue with tgPRK at a later time, since we are potentially removing a beneficial layer of the stiffer, cross-linked cornea, which helps to maintain the normalized corneal shape.
(4) By removing Bowman's membrane with the tgPRK element, there may be facilitation of the riboflavin solution penetration in cornea stroma, and less “shielding” of UV light in its passage through the cornea, causing more effective CXL.
While an ectasia case can have an improved visual result with the addition of the tgPRK procedure, completely removing significant refractive errors was not our goal. We have placed an arbitrary “ceiling” of 50 microns on the amount of tissue that we safely removed centrally, anticipating that further thinning might destabilize the cornea's biomechanical integrity, even following the “stiffening” effect of the cross-linking.
It should be noted that the proprietary riboflavin solution used was a slightly hypotonic (340 mOsm) formulation, resulting in slight “swelling” of the cornea intraoperatively (during the CXL). This restored the corneal thickness to a level near 400 microns during the time of CXL to protect the corneal endothelium; we did not en counter any corneal endothelial decompensation in any eyes studied herein despite treating cases under the theoretical limit of 400 µm corneal thickness12 prior to the CXL part of the procedure (Case 4).
In addition, the laser treatment was applied with caution, as the refractive effect of the CXL (cornea flattening) had to be anticipated. For this reason, we elected to always attempt a significant undercorrection of both the sphere and cylinder by at least 30%. At a later time, we hope to more accurately determine the new ablation rate of CXL-stroma.
Simultaneous tgPRK and CXL appeared to be effective in the rehabilitation of post-LASIK ectasia. The overwhelming reality of the efficacy of tgPRK and CXL has been the dramatic reduction of penetrating keratoplasty cases performed for the indication of KCN and post-LASIK ectasia in our practice over the last four years. The same-day, simultaneous tgPRK/CXL procedure was easy to perform, but in some cases the central epithelial surface took up to a month to regularize and become lucent. It took from one to four weeks for us to detect stable changes in the keratometry and topography, which seemed to match the visual and refractive changes.
Of course, the main goal for all refractive surgeons is to try to eliminate or at least significantly reduce the number of eyes developing ectasia after PRK and LASIK. We believe that there are eyes in which we cannot detect a pre-existing condition that may lead to ectasia following either PRK or LASIK. However, by eliminating eyes with abnormal preoperative topography and leaving corneas with the maximum clinically acceptable residual stromal thickness, we will definitely reduce the number of eyes developing ectasia.
Conclusions
Our findings, with significant long-term follow-up, suggest promising results with same-day, simultaneous topography-guided PRK and collagen cross-linking (the Athens Protocol), as a therapeutic intervention in highly irregular corneas with progressive post-LASIK ectasia. We are reporting herein for the first time effective CXL treatment in cases with minimal thickness under 350 microns. Our study demonstrates that we now may have another means of improving the visual and refractive results of a devastating complication while avoiding or delaying penetrating keratoplasty. OM
References
1. Binder PS. Ectasia after laser in situ keratomileusis. J Cataract Refract Surg. 2003;29:2419-2429.
2. Randleman JB, Russell B, Ward MA, et al. Risk factors and prognosis for corneal ectasia after LASIK. Ophthalmology. 2003;110:267-275.
3. Tabbara K, Kotb A. Risk factors for corneal ectasia after LASIK. Ophthalmology. 2006;113:1618-1622.
4. Klein SR, Epstein RJ, Randleman JB, et al. Corneal ectasia after laser in situ keratomileusis in patients without apparent preoperative risk factors. Cornea. 2006;25:388-403.
5. Binder PS. Analysis of ectasia after laser in situ keratomileusis: risk factors. J Cataract Refract Surg. 2007;33:1530-1538.
6. Hafezi F, Kanellopoulos J, Wiltfang R, et al. Corneal collagen crosslinking with riboflavin and ultraviolet A to treat induced keratectasia after laser in situ keratomleusis. J Cataract Refract Surg. 2007;33:2035-2040.
7. Kanellopoulos AJ. Post LASIK ectasia. Letter to the editor. Ophthalmology. 2007;114:1230.
8. Kanellopoulos A, Binder PS. Collagen cross-linking (CCL) with sequential topography-guided PRK. A temporizing alternative for keratoconus to penetrating keratoplasty. Cornea. 2007;26:891-895.
9. Ewald M, Kanellopoulos J. Limited topography-guided surface ablation (TGSA) followed by stabilization with collagen cross-linking with UV irradiation and riboflavin (UVACXL) for keratoconus (KC). Invest Ophthalmol Vis Sci. 2008:49:E-Abstract 4338.
10. Kanellopoulos AJ. Comparison of sequential vs same-day simultaneous collagen cross-linking and topography-guided PRK for treatment of keratoconus. J Refract Surg. 2009;25:1034-1037.
11. Kanellopoulos AJ. Managing highly distorted corneas with topography-guided treatment. ISRS/AAO 2007 Subspecialty Day / Refractive Surgery Syllabus. Section II: Ablation Strategies:13-15.
12. Spoerl E, Mrochen M, Sliney D, et al. Safety of UVA-riboflavin cross-linking of the cornea. Cornea. 2007;26:385-389.
| Transepithelial PRK and Collagen Cross-linking: Selected Case Reports |
|---|
| • Patient 1: Post-LASIK ectasia OU. Both eyes treated with tgPRK and CXL in one session. A 39-year-old male had LASIK elsewhere in May 2004. At that time, by patient history, the pre-operative UDVA (UCVA) was counting fingers OU. The manifest refraction was −6.50 −0.50x020 OD (20/20) and −6.00 0.50x165 OS (20/20). The preoperative keratometry readings and the corneal thickness readings were not available. There was no surgical data available. Following LASIK, the patient achieved an uncorrected vision of 20/20 in each eye, and reportedly plano refraction in both. In October 2005, he complained of progressively decreasing vision in both eyes. At that time, the UDVA (UCVA) was 20/50 OD and 20/40 OS; he reported that he was told that “astigmatism was developing.” The patient was seen by us in March 2006, 26 months after LASIK, with a manifest refraction of +2.25 −1.75x090 (20/20) OD and −1.25 −0.75x010 (20/20) OS. The UDVA (UCVA) was 20/40 OD and 20/30 OS. The keratometry readings were 38.75x90/ 35.62x180 (OD) and 40.65x05/39.55x95 (OS). The pachymetry readings were 495 µm (OD) and 505 µm (OS), respectively (Pentacam and ultrasound). A diagnosis of bilateral post-LASIK ectasia was made. Because of the decrease of the uncorrected vision and ectasia, the patient was presented with the risks, benefits and alternatives of the combined tgPRK/CXL technique, which was performed on both eyes in January 2007, 32 months after the LASIK surgery. Based upon the clinical manifest refraction of OD + 2.25 −1.75x90 (20/20) and OS −1.25 −0.50x005 (20/20), the attempted correction was reduced to + 1.75 −1.50x90 and −0.75 −0.50x005 for OD and OS, respectively. (The goal in the treatment was modified to anticipate the possible long-term flattening effect that CXL may have had on these corneas). In February 2010, 47 months after the tgPRK/CXL procedure, the UDVA (UCVA) improved to 20/40 OD and 20/20 OS with a manifest correction of −0.75 OD (20/20) and +0.25 −0.25x95 OS (20/20). The keratometry readings were 37.50x85/36.62x175 OD and 37.75x79/37.87x169 OS. Ultrasound pachymetry was 440 µm OD and 414 µm OS. Figure 1 demonstrates the pre- and post-tgPRK/CXL topography of the right eye as well as their difference map. 
Figure 1 summarizes the clinical course of the right eye in Case 1. The topographic image on the left shows marked central-inferior corneal steepening consistent with ectasia. The central image shows the final topography two years later. The topography here is flatter and quite normalized. The image on the right demonstrates the comparison (pre-treatment minus post-treatment). • Patient 2: Ectasia OU, OD treated, OS observed. A 33-year-old female reportedly had a manifest refraction of −4.00 −2.5x 90 OD (20/20) OD and −1.50 −2.00x100 OS (20/20). No other pre-operative data was available. The patient gave a history of eye rubbing. Sometime in 2002 the patient underwent bilateral LASIK surgery (the exact date is unknown and the surgical data were unavailable). Initially, the patient recovered excellent uncorrected vision, but in December 2005, over three years after surgery, she presented with a history of slowly decreasing vision in both eyes. At that visit, the UCVA was 20/800 in each eye. The manifest refraction was −10.50 −6.00x105 OD (20/40) and −7.75 −2.50x110 OS (20/30). The ultrasonic central corneal thicknesses were 395 µm OD and 410 µm OS. The keratometry readings were 52.87x103/46.12x13 (OD) and 47.12x111/45.00x021 (OS). Corneal topography revealed bilateral post-LASIK ectasia greater in the right eye. In December 2005, the patient decided to undergo the tgPRK/CXL only in the right eye, with no treatment in the left eye. At this time, manifest refraction was −10.50 −6.00x105 (20/30) in the OD and −7.75 −4.50x130 20/40 in the OS. In June 2007, 18 months after the tgPRK/CXL procedure, the UCVA was 20/800 in each eye. The manifest refraction in the treated right eye had worsened to −12.00 −2.50x100 (20/40). The keratometry readings were 48.00x29/47.3x119 (OD) and 47.87x 20/46.2x110 (OS), and the ultrasonic corneal thickness readings were 424 µm OD and 388 µm OS. The corneal topography revealed flattening in the difference map in the right eye (Figure 2). The patient was unhappy with this result and is currently uncomfortable with her anisometropia. She decided not to proceed with treatment in the fellow eye because she was unconvinced she had benefited from the tgPRK/CXL procedure. She is currently wearing rigid gas permeable contact lenses in both eyes. 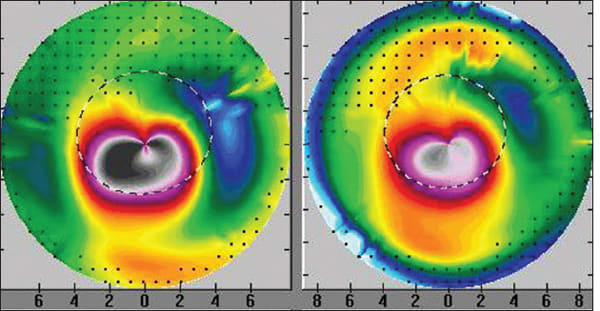
Figure 2. The topography on the left is prior to the tgPRK/CXL treatment with marked inferior steepening. The topography on the right shows the same cornea 16 months after tgPRK/CXL with marked flattening of the ectasia and cornea normalization. • Patient 3: Post-LASIK ectasia only in one eye, treated with tPRK/CXL in one session, the other eye observed. A 26-year-old male helicopter pilot underwent LASIK in both eyes in June 2004. None of the operative data was available to us. The only detail available for the initial LASIK procedure is that he was “about” −3.0 D myopic in both eyes prior to the LASIK surgery. His UDVA (UCVA) for the initial two years post LASIK was “good” and then deteriorated in his OD. He was subsequently diagnosed with ectasia and was offered Intacs or a corneal transplant. We first evaluated him in September 2007 three years after the LASIK surgery. The UDVA (UCVA) was 20/40 OD and 20/15 OSD. The manifest refraction was +1.50 −2.00x65 OD (20/20) and plano OS (20/15). The keratometry readings were 41.62x6/ 43.62x155 OD and 41.75/42.12x10 OS. The central ultrasonic pachymetry was 476 µm OD and 490 um OS. In September 2007, 39 months after the LASIK procedure, we performed a combined topography-guided PRK and immediate CXL in the OD for +0.50 −1.50x60. The planned laser resection was 35 µm. The pre-treatment manifest refraction was +1.50 −2.00x65; we reduced the attempted sphere and cylinder, anticipating a subsequent flattening effect of the sequential CXL procedure. Within six months the UDVA (UCVA) improved to 20/25; 24 months later in September 2009, the UDVA (UCVA) improved to 20/15 and the manifest refraction improved to plano −0.25x05 (20/10). The keratometry readings in the OD were 43.00/ 43.25x07 and the ultrasonic pachymetry was 441 µm. The difference maps (Pentacam) between the pre tgPRK/CXL and two years later are displayed in Figure 3. At three years follow-up, UDVA (UCVA) remains at 20/10. As a result of the improvement and stability of his visual function, this young man has recently went on to join the United States Air Force as a fighter pilot currently serving in active duty. 
Figure 3 summarizes the clinical course of the right eye. Topographic image 3A shows the topography of the right eye three years following the LASIK procedure with irregular astigmatism and marked inferior cornea steepening. UCVA 20/40 and BSCVA with +1.50 −2.00x65 20/20. Image 3B shows the topography three months after the tgPRK/CXL procedure. The topography here is flatter and quite normalized. Uncorrected visual acuity is 20/15. Image 3C is a topographic reproduction of the topography-guided PRK treatment plan with the Wavelight platform used. This platform is planning to remove tissue in an irregular fashion in order to normalize the cornea ectasia seen in 3A. Image 3D (A minus B) is a comparison map derived from subtracting image B from A and represents the topographic difference in this case three months after the combined treatment. The paracentral flattening is self explanator,y as the PRK and CXL have flattened the cone apex. The superonasal arcuate flattening is quite interesting. It represents the actual part-hyperopic correction that the topography-guided treatment has achieved, in order to accomplish steepening in the area central to this arc. Thus, the treatment has normalized the ectatic cornea by partially flattening the cone apex and at the same time by “steepening” the rest of the central cornea. • Patient 4: Use of tgPRK/CXL despite thin residual stromal bed. This 32-year-old female underwent LASIK in both eyes in December 2006 for a refractive error of −3.75 OD and −4.00 OS. No other data were available in regard to the surgery. The vision was good for two years and then started to deteriorate. Her treating surgeon diagnosed post-LASIK ectasia in December 2008. We first evaluated the patient in January 2009. The UDVA (UCVA) was 20/100 in the OD and 20/20-2 in the OS. The CDVA (BSCVA) was 20/30 with −3.25 −3.25x45 and 20/15 with + 0.50 −1.25x100, respectively. The keratometry readings were 46.7/ 44.1x66 and 39.75/41.75x65, respectively. The pachymetry readings were 419 µm and 460 µm, respectively. The diagnosis of post-LASIK ectasia was confirmed by Pentacam maps in the right eye (Figure 4, left image). The patient was contact lens intolerant and opted to undergo tgPRK/CXL despite the informed consent that the estimated residual cornea thickness would be 360 microns. This procedure was performed in February 2009 in the OD. 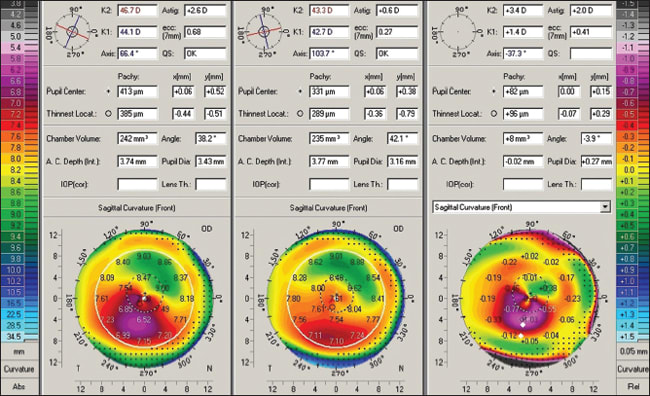
Figure 4. This is a Pentacam comparison of the right eye. The column on the left shows pre-tgPRK/CXL data and topography. The central column shows postop data and topography. The right column shows the difference of pre-treatment minus the post-treatment. The planned correction was −2.50, −2.50x45 after a 6 mm in diameter, 50 µm PTK. Using a moistened weck-cell, 0.02% MMC was applied after the ablation onto the stroma for 20 seconds. In January 2010 (11 months following treatment) the UCVA was 20/30, the CDVA (BSCVA) was 20/20-1 with −0.50 −0.75x141. Keratometry was 43.3/42.7x103. Pachymetry was measured at 330 µm. The difference map of pre and post treatment is noted in Figure 4. The endothelial cell count was unchanged at 20 months (2650 to 2600). The corneal OCT of the central cornea in the OD at 11 months is seen in Figure 5. The hyper-reflectivity of the anterior two-thirds of the cornea suggests the CXL effect, as we have reported previously, in applying similar treatment in keratoconus cases.10 The hypereflective demarcation in the middle of the cornea in this case suggests a very thick LASIK flap calculated to over 200 µm. 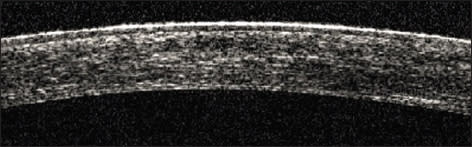
Figure 5. This is the OCT of the central cornea in the OD at 11 months post-tgPRK/CXL. The hyper-reflectivity of the anterior two-thirds of the cornea suggests the CXL effect. The hypereflective demarcation in the middle of the cornea suggests a very thick LASIK flap calculated to over 200 µm. |
| A. John Kanellopoulos, MD, is the medical director of the Laservision Institute of Athens, Greece, and a clinical professor of ophthalmology at New York University Medical College in New York City. He can be reached at ajkmd@mac.com. |








