Dry Eye Relief
Peeling back layers to reveal root causes, select the right tools and improve tear quality.
By Steven L. Maskin, MD, FACS
Many years ago, during a Grand Rounds on Cornea and External Diseases, a highly respected, and well known (noncornea) ophthalmologist stated to the audience of residents, fellows and other faculty that the care of dry, irritated eyes was so frustrating that he turned a deaf ear when confronted with these complaints. These sentiments were shared by many physicians then, and probably by many more today.
Much has changed in the evaluation and management of dry eye since that Grand Rounds over 20 years ago, including the recognition of dry eye as an inflammatory disease characterized by a hypertonic tear film created by an aqueous or evaporative tear deficiency.1 But practically speaking, how does a busy eye doctor use this information to help his patients, reversing symptoms and in some cases improving the patient's quality of life?
Here I will present a set of 13 points which have emerged as the basic approach I follow to manage these cases; revealing root causes and selecting the right tools to improve ocular surface health and reduce dry eye symptoms. Indeed the frustration of managing dry eye is in large part frustration with the inadequate level of understanding and treatment of meibomian gland dysfunction (MGD). Because my practice of over 20 years is limited to dry eye and nonrefractive cornea diseases, I've spent a great deal of time working on this most common and yet enigmatic cause of dry eye. Over 5 years ago, it became clear that important lessons about MGD were locked inside each gland just beyond our reach. I was compelled to find a nontraumatic way to unlock its secrets. I became focused on what has become in my practice, and subsequently in many others, a significant breakthrough in dry eye management and understanding of MGD: an in-office intraductal meibomian gland probing (MGP) procedure for obstructive MGD.
This procedure, developed in collaboration with Rhein Medical, establishes or confirms a latent duct system, leading to immediate and dramatic improvement in symptoms.2 This article will also review the latest results of MGP with and without intraductal microtube steroid injection as well as a qualitative classification system that I use to categorize meibum secreting lid functionality.
Revealing Root Causes
1. I recruit the patient as my partner with the shared goal of obtaining comfortable eyes. If the patient is not on board—understanding what we are doing and why—my job is ten times more difficult. I recommend patients read my book “Reversing Dry Eye Syndrome”.3 With a basic foundation of knowledge established, our office visits are more effective, which leads to faster results and reduced avoidable disease.
2. Making the diagnosis. The history is essential to unraveling the complaint. Sandy, gritty, grainy symptoms are usually aqueous tear deficiency (ATD), while burning and stinging is typically MGD. Allergy may cause burning as well as itching. Other common diseases, such as anterior blepharitis, conjunctivochalasis, floppy lid, delayed tear clearance and nocturnal lagophthalmos, may modify the presentation of disease, while increasing the severity of certain signs and symptoms. Together, the history and external examination may provide clues for an underlying systemic disease or regional predisposing or exacerabating disease, such as herpes zoster ophthalmicus or even greater occipital neuralgia (see “Don't Miss this Diagnosis” at the end). Slit lamp examination helps identify tear film and ocular surface diseases but also local ocular factors deeper than the surface, such as elevated intraocular pressure and microcystic corneal edema. With vital staining, tear break up time and fluorescein clearance test (FCT) results, I am able to develop an overarching hypothesis and list of diseases individualized for that patient and prioritized to level of impact. The FCT is very helpful in evaluating tear production, reflex tearing and delayed tear clearance.4
3. More on evaluation of MGD. There is a wide profile of MGD symptoms. In addition to burning and stinging there is also photophobia, foreign body from capped gland, plus lid stickiness, gumminess, pressure, heaviness, puffiness, awareness, itching, as well as generalized irritation and discomfort.
Perhaps the most interesting discovery about the evaluation of MGD is the frequency of lid tenderness, which does not seem widely appreciated. Our work on this disease suggests lid tenderness may be a marker of elevated intraductal pressure, more often noted on the upper than lower lids. Patients only occasionally complain about this, possibly because they've learned not to touch their lids. Yet this can be quite impressive with some patients recoiling from a gentle touch on the lid margin against the globe or with a finger or cotton-tipped applicator. It is important to differentiate lid tenderness from the simple sensation of pressure on the globe.
4. Qualitative classification of MGD based on lid tenderness and diagnostic expression. During the examination, every dry eye patient receives an evaluation of overall meibum secreting lid functionality based on characteristics and results of meibomian gland digital diagnostic expression for that lid. The major differentiation points for a lid are that of1 expressible meibum with at least five glands of a lid expressing meibum with mild digital pressure over the gland for 10 seconds5 and overall lid tenderness with direct mild pressure using a cotton-tipped applicator or finger.2
• Complete Proximal Obstruction and Complete Distal Obstruction. Lid tenderness may exist over the whole or part of a lid, from presumed elevated meibomian gland intraductal pressure secondary to obstruction with concurrent intact meibum production. We therefore refer to the glands within these lids as having a complete obstruction. If a gland shows expressible meibum, the complete obstruction must be deeper in the lid, which is proximal to the distal glands allowing some meibum to be expressed. If this tender lid has five glands expressible, then we describe it as “complete proximal obstruction or CPO (Figure 1).” If no meibum is expressible from at least five glands in a lid, the glands in the lid have a “complete distal obstruction or CDO” (Figure 2), indicating the complete obstruction is near the level of the orifice and glands are not able to secrete meibum to reach the orifice and lid margin. On lid transillumination, CPO could lead to short glands and loss of acinar-ductal units while CDO could lead to dilated ducts, and cystic acini with subsequent diffuse acinar atrophy and ultimate loss of whole glands.

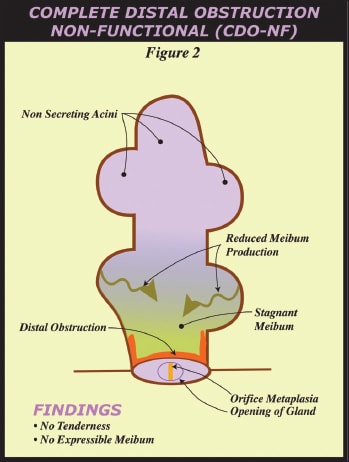
• Partial Distal Obstruction and Complete Distal Obstruction-NonFunctional. Lids without tenderness may or may not have at least five glands with expressible meibum. If a lid has at least five glands with expressible meibum and orifice metaplasia, but no lid tenderness, it is described as having a “partial distal obstruction or PDO” while a lid without expressible meibum from 5 glands and no lid tenderness is a “complete distal obstruction-nonfunctional or CDO-NF. In this case, meibum is not expressible so the obstruction is complete and distal. It is proposed that the glands are not generating meibum sufficient to elevate intraductal pressure causing lid tenderness.
5. Don't miss an infection! When in doubt, I like to culture lids, conjunctiva and lacrimal sac discharge and place in my office incubator. This way, I usually know the next morning if there is occult infection. Positive cultures are sent to the lab for identification and sensitivities. Demodex infestation should not be missed as it is symptomatic in some patients and use of tea tree products can bring it under control,6 so if I see a cylindrical deposit, I will epilate and look for the parasite under a light microscope.

6. Simplify the complexity. First, I create the overarching hypothesis to explain all of the symptoms and signs. Second, I prioritize the list of disorders for clinical impact on the patient. I simplify this list into systemic, regional and local (whole globe and surface problems) components and discuss the list with the patient. As these co-morbid diseases are discussed, I show how each is interwoven and impacts the others. I keep things simple and explain that we will target the top few problems first, peeling them back to reveal residual symptomatic disease to target. It is important for the patient to understand that it is a gradual process of obtaining comfortable eyes, it's not as simple as flipping a switch.
7. Initiate targeted therapy and adjust on follow-up visits. Based on the overarching hypothesis, the high impact diseases are treated first. During follow up, if the hypothesis and prioritization is accurate, then the patient's symptoms will have significantly improved. Depending on the level of improvement, I may or may not need to add further treatment or begin therapy for the other diseases. If there has been no improvement and the patient has been compliant with therapy, then a new hypothesis is required. Treatment continues until the patient is comfortable.

8. Be ready to treat for symptoms even with a lack of signs. Dry eye may have minimal or no obvious signs and yet patients may suffer greatly from tear deficiency. Symptoms may be out of proportion to the signs. For example, patients with dry eye after LASIK may commonly present without surface vital staining yet with real symptoms and suffering. If the exam, vital staining and FCT are all normal, the most common cause of irritation may be nonobvious MGD.7 Sometimes delayed tear clearance on fluorescein clearance test without aqueous tear deficiency presents with irritation and only mild hyperemia. This may respond to topical loteprednol (Lotemax, Bausch + Lomb).8
9. The less drops, the better. A common experience between managing glaucoma and dry eye patients is noncompliance with using prescribed drops. At some point, ocular surface nerves may lose sensitivity, which makes it easier to go without topical artificial tears. If ATD patients are having difficulty complying with 3 or 4 times/day tear supplements in the face of significant surface erosions, I will move to punctal occlusion. I prefer thermal cautery as the quickest and best way to restore increased tear volumes. Some patients prefer using cyclosporine (Restasis, Allergan) over punctal occlusion, which may be offered later if needed. Other therapies I use frequently for ATD include topical steroid,8 oral cholinergic agents such as cevimeline (Evoxac, Daiichi Sankyo) and autologous serum, in addition to the basics of environmental control and omega-3 fatty acid supplements.
10. Punctal occlusion works great for ATD. Before performing punctal occlusion, try to minimize surface inflammation. Press on the lacrimal sac and canaliculi to rule out an occult dacryocystitis or canaliculitis. Many colleagues successfully use plugs to punctal occlude, but I have found problems including frequent loss of plugs, local tissue trauma with nasal bulbar erosions, colonization with bacteria with intermittent seeding of the ocular surface, and fibrosis within the canaliculus. Plug proponents like its reversibility (assuming it does not scar into place), although I frequently use light cautery to create a superficial, sometimes partial, occlusion to maintain reversibility.
11. There is a paradigm change under way in the treatment of MGD.9,10 Traditional therapies that are helpful but show inconsistent results have included warm compresses with lid hygiene, massage, doxycyline and more recently, omega-3 fatty acids, Restasis and azithromycin (AzaSite, Inspire Pharmaceuticals). However, none of these interventions are able to render a gland free of obstruction. Without physically entering the gland, we can only speculate as to duct patency. Even when a gland shows meibum upon digital diagnostic expression, MGP has shown there can be obstruction deeper in the lid (or more proximal in the gland) allowing for the distal glands to secrete meibum through the orifice (CPO, see above). As noted, MGP has given us a successful, safe and well-tolerated procedure to establish and confirm ductal patency using intraductal probes (no adverse sequelae with longest follow-up of 43 months).12


• Results of MGP studies. Exam findings of lid tenderness suggest elevated IDP whose secondary effects are consistent with histopathology studies showing dilated ducts, cystic acini, plus acinar squamous metaplasia and atrophy.11 MGP has been shown to reduce 75-90% of LT symptoms, increasing to 95% reduction with adjunctive use of intraductal microtube steroid injection (MGD-S). Symptoms, excluding lid tenderness of MGD such as burning, stinging, and photophobia, improve with MGP to between 60 and 80% depending on the severity of signs/symptoms and co-morbid disease as well as use of MGD-S.12,13 MGP has also been shown to restore meibum-producing gland functionality in 26 of 27 lids (97%) in 15 patients, including one patient with graft vs host disease and tarsal fibrosis.14
12. The future is even brighter for treatment of MGD. Now that we can safely and successfully establish patent MG ducts, we can see the day that pharmaceutical therapy injected through intraductal microtubes and applied topically will modulate dysfunctional ductal and/or acinar epithelium. In the case of complete acinar atrophy, we can contemplate the regenerative potential of stem cells to repopulate the gland, then use pharmaceuticals to direct regeneration of in vivo functional glands.
13. Other treatments. Other treatments include ocular surface reconstruction using amniotic membrane for chalasis and superior limbic keratoconjunctivitis. Allergies are usually managed successfully with topical allergy therapy, irrigation with sterile preservative-free saline and a low-dose steroid as needed. Compounded topical cyclosporine may also be used in some cases. Nocturnal lagophthalmos is also common and I like to use the Onyix sleep mask (eyeeco.com), which is especially useful in the setting of continuous positive airway pressure use for sleep apnea.
Achieving success in managing dry eye and ocular irritation requires an open mind to peel back the layers of systemic, regional and local eye disease to develop an overarching hypothesis and list of diseases individualized for that patient and prioritized to level of impact. Subsequent successful treatment with the right tools will confirm the root causes leading to better tears, better ocular surface health and less symptoms. ■
| Do Not Miss This Diagnosis! |
|---|
| The diagnosis of greater occipital neuralgia and referred pain to the orbit and globe is frequently missed, but should always be on the differential of ocular irritation and pain. The impact of this diagnosis may best be displayed through the case of a 24-year-old college student from Ohio who had ocular involvement of juvenile rheumatoid arthritis that was being treated by a variety of eye doctors. She had poor vision due to an opaque cornea and was sent to me to perform a cornea transplant, which improved her sight. After a couple of months, she developed excruciating pain in the post-op eye without any findings on exam. No therapy would reduce her suffering. Unable to tolerate the pain, she asked her doctors to enucleate the globe. One week before the planned surgery, she returned to me for a transplant follow-up visit. The complaint of vague periorbital pain led me to evaluate the occiput, which was severely tender and painful upon palpation. Most importantly, palpation of her occiput caused the recreation of the identical pain in and around the left eye. To test my hypothesis, I injected lidocaine into the tight muscles in the occiput. The pain was immediately relieved in both her occiput and eye. The young lady was amazed, and shaken that she could have sacrificed her eye needlessly. She was treated successfully with heat, massage and muscle relaxants. |
References
1. The definition and classification of dry eye disease: Report of the Definition and Classification Subcommittee of the International Dry Eye WorkShop (2007). Ocul Surf. 2007;5:75-92.
2. Maskin SL. Intraductal meibomian gland probing relieves symptoms of obstructive meibomian gland dysfunction. Cornea. 2010;29:1145-1152.
3. Maskin SL. Reversing Dry Eye Syndrome: Practical Ways to Improve Your Comfort, Vision and Appearance. 2007 Yale University Press; New Haven, CT.
4. Maskin SL. Effect of ocular surface reconstruction by using amniotic membrane transplant for symptomatic conjunctivochalasis on fluorescein clearance test. Cornea. 2008;27:644-649.
5. Korb DR, Blackie CA. Meibomian gland diagnostic expressibility: correlation with dry eye symptoms and gland location. Cornea. 2008;27:1142-1147.
6. Gao YY, Di Pascuale MA, Elizondo A, Tseng SC. Clinical treatment of ocular demodecosis by lid scrub with tea tree oil. Cornea. 2007;26:136-143.
7. Blackie CA, Korb DR, Knop E, Bedi R, Knop N, Holland EJ. Nonobvious obstructive meibomian gland dysfunction. Cornea. 2010;29:1333-1345.
8. Pflugfelder SC, Maskin SL, Anderson B, et al. A randomized, double-masked, placebo-controlled, multicente comparison of loteprednol etabonate ophthalmic suspension, 0.5%, and placebo for treatment of keratoconjunctivitis sicca in patients with delayed tear clearance. Am J Ophthalmol. 2004;138:444-457.
9. Maher, I. Procedure helping clear up chronic dry eye. St. Petersburg Times, Fla., August 11, 2010.
10. Stern GA. Blepharitis that won't go away. American Academy of Ophthalmology Subspecialty Day 2010 Lecture.
11. Obata H. Anatomy and histopathology of human meibomian gland. Cornea. 2002;21:S70-S74.
12. Maskin SL. Intraductal meibomian gland probing and distinct visual analog scale response profiles for symptoms of meibomian gland dysfunction. Poster presentation AAO Chicago 2010.
13. Maskin SL Intraductal meibomian gland probing with adjunctive intraductal microtube steroid injection for meibomian gland dysfunction. Poster presentation during ARVO, Fort Lauderdale, Fla., 2011.
14. Maskin SL. Intraductal meibomian gland probing to restore gland functionality for obstructive meibomian gland dysfunction. Submitted to the American Academy of Ophthalmology for release during annual meeting in Orlando, 2011.

|
Steven L. Maskin, MD, FACS, is the medical director of the Dry Eye and Cornea Treatment Center in Tampa, Florida. His practice focuses exclusively on dry eye and related diseases with special interest on meibomian gland dysfunction. Dr. Maskin has patents pending on intraductal probing diagnosis and treatment apparatus as well as jojoba treatment solutions for MGD. |








