Special Section Sponsored By Optos
Exploring the Ultra-widefield View
A widefield view of the retina is changing our understanding of disease and enhancing its diagnosis and management.
By Erin Murphy, Contributing Editor
Imaging technologies continually push the boundaries of a simple goal: to see more. Physicians continually strive to put that greater vision to practical advantage in diagnosing patients and making treatment decisions – to do more.
At this year's American Academy of Ophthalmology meeting in Chicago, physicians got their first look at the Optos 200Tx, the first imaging device to offer an ultra-widefield view of autofluorescence changes to retinal pigment epithelium (RPE). What they saw were simultaneous non-contact pole-to-periphery views of the retina (up to 200° or 82% of the retina), combined with fluorescein angiography and autofluorescence capabilities.
The device will be available in 2011, but several physicians who are already using it shared their experiences and discussed its role in diagnosing, analyzing, documenting and monitoring ocular pathology in the retinal periphery.
Advancing autofluorescence
As RPE cells accumulate materials that include intrinsic fluorophores, autofluorescence imaging helps detect and visualize those materials, according to Frank Holz, MD, professor and chairman of the Department of Ophthalmology at the University of Bonn in Germany. Autofluorescence imaging shows a disease pathway contained in living cells, relevant in many retinal diseases. Physicians can observe the topographic distribution of increased signal, which may indicate a diseased retina, or reduced signal, possibly due to dead retina with loss of RPE cells containing the dominant intrinsic fluorophores.
“The Optos 200Tx is a wonderful instrument. I think it will expand not only the broader view on the retina but also the clinical application of fundus autofluorescence imaging,” says Dr. Holz. “We can see both macular pathology and differences in autofluorescence signals in an expanded view.”
Autofluorescence imaging is helpful in diagnosing disease, understanding pathogenesis and monitoring progression in diseases such as AMD.
“There's a growing spectrum of clinical applications for which we look at not only the diseased RPE, but also fluorophores that come up anterior to the RPE,” Dr. Holz explains. “We can identify predictive imaging biomarkers for disease — especially in dry AMD — and create new targets to interfere with disease processes based on these observations.”
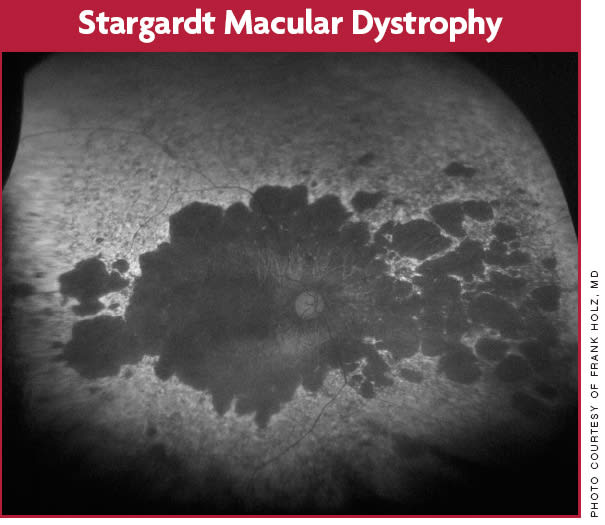
This patient with autosomal recessively inherited Stargardt disease shows multiple areas of outer retinal atrophy not only at the posterior pole but also extending peripherally.
Dr. Holz says autofluorescence imaging is informative in patients with other diseases as well. It also shows the pattern of luteal pigment (lutein and zeaxanthin) distribution, which may be altered with disease. Furthermore, autofluorescence imaging is helpful in identifying outer retinal atrophy in various diseases, including monogenetic diseases, such as Stargardt's or Best's, and complex, multifactorial diseases.
Autofluorescence imaging has been accepted by regulatory authorities as an anatomical endpoint to monitor geographic atrophy progression in AMD and to see if interventions are successful in slowing the marching death of atrophy,” Dr. Holz says.
Applying autofluorescence
Srinivas Sadda, MD, Associate Professor of Ophthalmology at the Doheny Eye Institute at the University of Southern California, is also impressed with the Optos 200Tx. “Even in some patients with nonmydriatic pupils, you can still obtain ultra-wideview peripheral images,” he says. “It's really quite spectacular.”
But Dr. Sadda wanted to go beyond spectacular images to gauge how he could apply them to diagnosing and treating patients. Do peripheral abnormalities have some characteristic autofluorescent patterns that correlate to disease, which can help ophthalmologists diagnose and manage patients?
Dr. Sadda and his colleagues sought to answer that question in a retrospective analysis of 122 patients (225 eyes). They evaluated autofluorescence images for the presence of abnormalities in the center and compared those images to the presence of abnormalities beyond the central degree.
“Once we computed the frequency of abnormalities in the periphery, we categorized patients by disease. We looked for patterns that repeated for a given disease to determine whether information from the periphery was particularly useful,” says Dr. Sadda. Diseases included AMD, inflammatory infectious diseases, retinal degenerations and ocular tumors.
“We're continuing to evaluate the results, but we've been very excited by the initial data,” Dr. Sadda says. “Peripheral abnormalities beyond the central 30° were present in 77% of the eyes in this series.”
Dr. Sadda and his colleagues found that the information gathered from the periphery didn't simply repeat the diagnosis that one could make from the ophthalmoscope alone. “This is a new avenue of information,” he says. “We're really struck by the fact that unusual autofluorescence patterns may ultimately aid us in diagnosis and classification.”
Even in the many cases where researchers correlated the abnormalities with areas of RPE disturbance, either on color photography or ophthalmoscopy, they often found that the abnormalities were more striking in autofluorescence.
“The contrast in autofluorescence is very helpful, and it's helping us begin to identify certain patterns that appear to be pathognomonic and characteristic for disease,” he explains. Already, it seems as though some of these patterns may only be clear in ultra-wideview images, not standard 30° images. “Patients with posterior uveitides, for example, had some autofluorescence abnormalities beyond the posterior pole. Without widefield imaging, you don't realize that these patterns are forming. There is useful information — and more to learn — in the periphery.”
Utilizing fluorescein angiography
In addition to the autofluorescent modality, early users of the Optos 200Tx are exploring its fluorescein ultra-widefield angiography capabilities. Szilard Kiss, MD, Director of Clinical Research at Weill Cornell Medical College, presented a study at the AAO meeting called “Ultra-widefield Angiography Significantly Improves Detection and Classification of Diabetic Retinopathy.” The purpose of the study was to assess the utility of ultra-widefield angiography in diabetic patients and compare it to the idealized seven standard fields used in the Early Treatment Diabetic Retinopathy Study (ETDRS).
The retrospective review evaluated 145 diabetic patients (218 eyes) who had undergone ultra-widefield angiography. Researchers examined areas of neovascularization, ischemia and non-perfusion, which were quantified by two masked graders and reviewed for discrepancies. Dr. Kiss and his colleagues found that ultra-widefield angiography represents a significant improvement in visualizing retinal pathology. It influences how they classify diabetic retinopathy and how they manage patients with the disease.
“I really couldn't imagine treating or taking care of these patients without the information of peripheral retina,” says Dr. Kiss. “There's definitely pathology in the periphery that will influence the way I follow my patients and may influence how I treat these patients. Even when we see neovascularization and know that we'll use the laser, images of the periphery may help us decide when to treat. Can we laser this before it reaches the threshold of vitreous hemorrhage? What about lasering this before we get the pathology in the ETDRS seven standard fields? If I see more pathology in the periphery, I might treat sooner.”
Dr. Kiss was able to visualize macular ischemia or macular edema occurring in the posterior pole, which is why he always uses ultra-widefield angiography for diabetic patients and increasingly for uveitis patients as well. Additional pathology is often present in the periphery. “Just from my experience, I'd estimate that greater than 85% of patients with neovascularization have non-perfusion or ischemia in the periphery, and it's usually very striking,” he says.
Taking a wider view
The Optos 200Tx fulfills its purpose to show doctors more of the retina. What's more, as it offers more information, physicians are learning how diseases manifest themselves in the periphery. Already, exciting new views and patterns are emerging to influence patient follow-up and treatment. Soon, more data will become available through the Age-Related Eye Disease Study 2 (AREDS2), in which the 200Tx will be used to determine the frequency and significance of peripheral retinal abnormalities in an effort to curtail vision loss related to AMD progression — an additional step from seeing more to doing more.








