Neuroadaptation & Premium IOLs: What Does the Brain Think?
A look at the science behind the lens.
By Robert M. Kershner, MD, MS, FACS
The evolutionary history of the primates that predate us can be traced back more than 65 million years. That is a significant period of time in which to evolve sensory systems that have assured our survival. So how could such a complex structure as the eye have evolved by chance? Even Darwin acknowledged that the eye did not fit well into his theory. Fortunately, the development of the eye itself corresponds to every stage of development that we have uncovered in every existing living species.
The first animal with a primitive eye lived more than 550 million years ago. According to scientific calculations, that would mean only 364,000 years was necessary for our camera-like eye to first appear. If primate vision required a “perfect” optical apparatus in order to survive, then today we would all be emmetropic. Yet we are not. Our vision evolved in an unusual way using neural adaptation to compensate for and enhance the visual process.
So how does the brain react when something artificial, like a premium IOL, is implanted into the eye? This article will explain the adaptation mechanism and how new technological developments make the process more successful.
The Changing Brain
The adult nervous system is remarkably plastic and its ability to modify input is quite rapid. Through this process of neuroadaptation, the brain modifies its sensory input to gain a survival advantage. It is a fundamental component of sensory information processing. Neuroadaptation can take as little as a tenth of a millisecond or a few minutes to occur, and is experienced with regularity within the visual system. An illustration of this remarkable plasticity is Adam Kohn's demonstration of a perceptual reduction in contrast when viewing a high-contrast pattern of vertical bars (by rapidly moving your eyes across the pattern to prevent retinal afterimage) and then quickly looking at a similar pattern in a subsequently viewed stimulus and seeing a reduced low-contrast image (Figure 1). This aftereffect does not reduce sensitivity to the original contrast pattern, suggesting that the appearance of the bars is orientation and spatial-frequency specific.
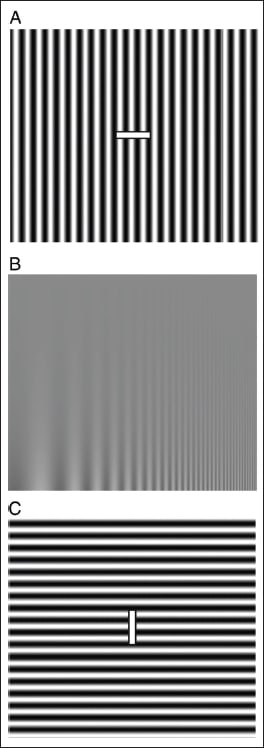
REPRINTED WITH PERMISSION FROM KOHN, AJ. NEUROPHYSIOL 2007;97:3155-3164. |
Figure 1. Perceptual reduction in apparent contrast. Slowly move your eyes back and forth (to prevent retinal afterimages) along the white bar at the center of A for 30 seconds, then transfer your gaze to the test image in B. A low-contrast portion of the image (top) should briefly appear invisible. Adaptation with the pattern in C does not reduce sensitivity to the test pattern, demonstrating the orientation specificity of the effect. Note that the aftereffect is also spatial frequency specific: lower (left) and higher (right) spatial frequencies in the test image are not affected by adaptation with A. |
Where is Neural Integration And Adaptation Occurring?
Neural adaptive roles in the consolidation of memory, emotion, addictive behaviors, navigation and spatial orientation are all linked to the hippocampal region of the brain. There is no question that this region plays a crucial role in neuroadaptation. However, virtually every area of the cerebrum, including the visual cortex, plays a part.
Normally, a visual stimulus will traverse from the ipsilateral geniculate nucleus to the primary visual cortex (V1) of each occipital hemisphere by splitting into two primary pathways — the so-called dorsal stream and the ventral stream. The dorsal stream travels first into V2, then onto the dorsomedial area and V5 before it ends up in the posterior parietal cortex.
For example, stimuli traveling in the dorsal stream will require adaptation of neurons in cortical area MT (V5), an extrastriate visual area containing a high proportion of neurons that are selective for the direction of motion and an object's location. This information is used to control hand-to-eye coordination.
Contrast Adaptation
Neuroadaptation is a cortical neuron response's ability to be altered by a recent stimulus. Unlike light adaptation, which occurs entirely in the retina, contrast adaptation begins at the earliest stages of the visual system — the retinal ganglion cells — and then evolves to include wide areas of the cerebral cortex.
You probably learned as a child that prolonged viewing of a moving stimulus while looking at a static image makes the non-moving image appear to move in the opposite direction (the well-known motion aftereffect, MAE). Models of visual motion processing suggest that some form of opponent processing is occurring where the response to stimuli moving in one direction is subtracted from those moving in the opposite. Contrast sensitivity and its relationship to motion occur within the same brain areas and are critical to important daily tasks such as driving an automobile.
Diffractive and Refractive Technology
Most multifocal IOLs in use today are either diffractive, refractive or a combination of both principles. Diffractive multifocal IOLs are pupilindependent and sacrifice intermediate vision by focusing incoming light rays at two points either near or far. Refractive multifocal IOLs are pupildependent, incorporating different refractive zones to create focal points at varying distances. Many patients with refractive IOLs find vision at intermediate distances better, but have difficulty with near. Lens manufacturers have used both optical principles, such as in the combined apodized, diffractive-refractive IOL, and others are developing newer multizonal and progressive lens designs in an effort to address these limitations.
What role does neuroadaptation play when a patient is faced with alterations to the visual system induced by the new premium IOLs? These lenses do not replicate the natural state. Just as patients have trouble adjusting to their first pair of progressive add eyeglasses, premium IOL patients will be challenged by their perceptive change. How quickly and how well a given patient adapts to this change will determine if they are satisfied with the surgical result or not. It is incumbent upon the surgeon to take the time to thoroughly discuss the implications of this change with respect to the patient's day to day needs before the patient undergoes surgery. Inquiring as to which visual tasks a patient is looking to improve can go a long way to improve satisfaction.
Particular attention needs to be placed on patient education of the process of neuroadaptation and possible training techniques to increase awareness. Patients have high expectations when it comes to time and demand immediate results. If they are told ahead of time to expect a gradual adaptation period following their surgery, they will most likely accept this prospect. If the patient is impatient, it might be better to forego the premium IOL altogether and go with one that provides goof-proof results. Patients are always more likely to be accepting if their surgeons explain in terms they can understand before surgery day, address specific surgical needs of the patient during the procedure and forewarn of what the patient can expect visually after the surgery.
I have previously investigated and published the role of IOL optics in image contrast sensitivity. Our findings compared the effects on retinal imaging and functional visual performance of an aspheric intraocular lens (Tecnis Z-9000) with those of conventional spherical optics — silicone (AA4207VF) and acrylic (AcrySof SA60AT). With functional acuity contrast testing, we found that use of an aspheric IOL creates a measurable 38-47% increase in photopic and a 43-100% increase in mesopic visual performance.
We know that improvement in visual perception is due in large part to the improved optics of the aspheric IOL, which corrects for the cornea's positive spherical aberration. Analyzing digital fundus images taken through these lenses corroborated this further. What we did not know then, but know now, is that at least some of the improved visual quality can be attributed to the neuroadaptation process — something much more difficult to analyze objectively.
Visual Disturbances
Neuroadaptation can occur within the visual system in response to either a monocular or binocular visual disturbance. Visual adaptation depends to a great extent on visual awareness. In the case of a monocular visual disturbance, the brain learns to compensate by altering its perception. It has been shown that even in cases where a clear image is focused onto the retina, neuroadaptation may have to kick in if there are inherent optical aberrations within the visual system that the brain cannot accept.
Given time, the mind applies its negating effect to the undesirable pattern. If age and time work in the patient's favor, then the final image ultimately becomes acceptable. However, sometimes surgeons intentionally disrupt the “one-eye, one-image” perception that is required for successful merging of the images from two eyes, such as when implanting an IOL style for one eye that is different from the other. In this case, the brain is presented with a perceptive paradox that it is not wired to undo.
The study of neuroadaptation is based primarily in psychophysics. Two extensively studied phenomena are known as binocular rivalry and visual crowding. These visual phenomena are capable of erasing visual stimuli from conscious awareness. Unlike factors that lead to visual processing early in the system, processing of these phenomena occur within the primary visual cortex (V1) and the middle-temporal visual areas. Brain imaging and EEG studies have demonstrated that suppression of unwanted images during retinal rivalry reduce the visual stimuli perceived in the monocular regions of V1 and keep them from conscious awareness. Research in this area suggests that suppression of vision rivalry and crowding involves a reduction of neural activity, not an increase or elimination.
How the brain recruits the neurons to make this happen is just beginning to be understood. Every processing point along the visual pathway contributes to the final clearly perceived optical image, and an interruption in the smooth flow of information anywhere in the visual stream can become problematic. Until the image signal hits the sixthorder neurons, both images are monocular. It is here where ocular dominance and retinal rivalry exist. From the lateral geniculate bodies, the images begin to fuse. Flood these centers with retinal signals from multiple images and the deep centers of the brain that need to make sense of the chaos begin to fail. Like contrast, neural adaptation associated with both retinal rivalry and image crowding occur at the earliest stages of visual processing.
When reading, our eyes move in spurts across the page. To meld the saccadic movement of our eyes into a smooth perception of letters and words requires higher cortical processing. The brain adapts to the information from these images and combines them across glances. If, during the course of our lives, we lose the ability to modulate visual in formation, such as what occurs following a stroke, retraining the brain to perceive visual stimuli differently is necessary.
Conclusion
How do we integrate what we have learned from neurophysiological study into an understanding of the neuroadaptative processes in our patients as we modify a lifetime of visual perception with one quick stroke of a diamond blade? Perhaps we can garner some information from the work done with the visual rehabilitation of stroke victims. In these cases, patients have normally functioning retinas, ganglion cells and optic nerves, yet pieces of the image cannot reach the optical centers of the brain that are needed to process them due to ischemic neuronal damage. Patients with chronic visual field defects can be rehabilitated with a computer-based program over a three-month period. Objective studies show that the visual-evoked response and positron emission tomography imaging (PET scan) improve both functionally and clinically following rehabilitation.
Can we truly screen our patients for those most likely to benefit from premium IOLs and avoid those who will not? There is reason to believe that patient-profiling data based upon personality, risk-taking behavior and visual demands may help. The pleasure-seeking areas of the brain (limbic system, amygdala, thalamic nuclei) are in close proximity to the neuroadaptative centers of the brain (hippocampus). Most surgeons would probably avoid operating on gamblers, drug addicts and bungee jumpers. However, one hypothetical approach worth investigation might be to start our patients on dopamine prior to surgery.
We surgeons should ask ourselves some questions before we cut. If we can retrain a brain whose optics have changed, how best should it be done? Should we delve into the cortical abilities of our patients? Can manufacturers purposefully design optics to enhance an individual's interpretation of visual input? The answers to these questions are not simple. Solutions to the problems we face will not be easy. What is certain is that an assessment of our patients' behavioral, social and psychological needs are at least as important as the measurement of their acuity and A-scan. OM
Bibliography
1. Kershner, RM. Neuroadaptation. Mastering Refractive IOLs-The Art and Science. David Chang, MD, editor. Slack, Inc. Thorofare, NJ. 2008. Ch 79 pp 302–304.
2. Kershner, RM. Patients' NeuroAdaptive qualities may predict surgical success. Ocular Surgery News. March 2008.
3. Kershner RM. Patient's adaptation to cataract surgery. Ophthalmology. 1998;105(1):6-7.
4. Misano J, Hardten DR, Kershner RM, Holladay JT, McDonald JE. Role of neuroadaptation with use of multifocal IOLs merits more discussion. Ocular Surgery News, U.S. Edition. 24(12):60 February 25, 2008.
5. Kent C, Kershner RM, Mainster M, McDonald JE, et al. Multifocal neuroadaptation: can training help the brain? Rev of Ophthalmol. XVII:(3):24–31 March 1, 2010.
6. Phillips P. New lens, same brain: the importance of neuroadaptation. EyeNet Magazine. July/August 2007.
7. Javitt JC, Steinert RF. Cataract extraction with multifocal IOL implantation: A multinational clinical trial evaluating clinical, functional and quality of life outcomes. Ophthalmology. 107:2040–2048, 2000.
8. Artal P, Guirao A, Berrio E, Williams DR. Compensation of corneal aberrations by the internal optics in the human eye. J Vis. 2001;1:1–8.
9. Webster MA, Georgeson MA, Webster SM. Neural adjustments to image blur. Nat Neurosci.2002;5:839–849.
10. Artal P, Chen L, Fernández EJ, et al. Neural compensation for the eye's optical aberrations. J Vis. 2004;4:281–287.
11. Kim CY, Blake R. Trends Cognit. Sci. 2005;9:381–388.
12. Tong F, Engel SA. Nature. 2001;411:195–199.
13. Blake R, et al. Strength of early visual adaptation depends on visual awareness. Proc Natl Acad Sci U S A. 2006, 103(12): 4783–4788.
14. Melcher D. Predictive transfer of visual adaptation before saccadic eye movements. J Vis. 2007;9:1003.
15. Jacobs GH, Nathans J. Color vision: how our eyes reflect primate evolution, Scientific American. March 16, 2009.
16. Kershner RM. Retinal image contrast and functional visual performance with aspheric, silicone, and acrylic intraocular lenses-prospective evaluation. J Cat Refract Surg. September 2003; 29:1684–1694.
17. Kershner RM. Contrast sensitivity and functional visual performance. Wavefront-Designed IOLs to Improve Functional Vision. Slack, Inc., pp.5–7, June 1, 2004.
18. Kershner RM. Improved functional vision with a modified prolate IOL. Clinical & Surgical Ophthalmology. March 2005.
19. Kohn A. Visual adaptation: physiology, mechanisms, and functional benefits. J Neurophysiol.97:3155–3164, 2007.
20. Adelson EH, and Bergen JR. (1985). Spatiotemporal energy model for the perception of motion. J. Opt. Soc. Am. A 2, 284–299.
21. Julkunen L, Tenovuo O, Vorobyev V, Hiltunen J et al. Functional brain imaging, clinical and neurophysiological outcome of visual rehabilitation in a chronic stroke patient. Restor Neurol Neurosci. 24(2):2006.

|
Robert M. Kershner, MD, is President and CEO of Eye Laser Consulting. He is a professor of Anatomy, Physiology and Microbiology at Palm Beach State College in Palm Beach, Florida. He has written extensively on neuroadaptation and the surgical correction of astigmatism. He may be reached via e-mail at kershner@eyelaserconsulting.com. |








