Rx Perspectives
How Drug Concentration Influences Antibiotic Efficacy
A review of fluoroquinolone pharmacokinetics reveals the unique attributes of levofloxacin 1.5%.
By Marguerite B. McDonald, M.D., F.A.C.S.
The current generation of topical fluoroquinolones offer broad-spectrum coverage against ocular pathogens, and all three (levofloxacin, gatifloxacin and moxifloxacin) are widely used in ophthalmology. Recently, levofloxacin became available in a 1.5% concentration, estimated to be at least 3 times greater than that of other topical fluoroquinolones. This high concentration gives it exceptional tissue absorption and penetration, which, in turn, creates high tissue concentrations that persist longer than other members of its class. These sustained high drug concentrations, I will argue, make levofloxacin 1.5% a particularly effective agent and my drug of choice for many antibiotic applications.
| Marguerite B. McDonald, M.D., F.A.C.S., is a cornea/refractive/anterior segment specialist with Ophthalmic Consultants of Long Island in Lynbrook, NY, a clinical professor of Ophthalmology at New York University in Manhattan, and an adjunct clinical professor of ophthalmology at Tulane University School of Medicine in New Orleans. She is also a staff physician at both Manhattan Eye, Ear and Throat Hospital and the Island Eye Center of Carle Place, NY. Disclosure: Dr. McDonald has served as a clinical investigator for Vistakon Pharmaceuticals, the sponsor of the studies discussed in this article. |
Measuring Fluoroquinolone Potency
Fluoroquinolones work by binding to DNA gyrase and topoisomerase IV, two enzymes essential for bacterial reproduction. Inhibiting these enzymes halts bacterial replication. Differences in the binding affinities of the various fluoroquinolones account for much of the variation in minimum inhibitory concentration (MIC) between agents.
MIC is a useful measure of antibiotic potency; it is defined as the lowest concentration of drug that will inhibit growth of a given percentage — typically 50% (MIC50) or 90% (MIC90) — of isolates of a specific organism. MIC is strictly an in vitro measure, and, as we shall see, MIC values are most meaningful when used in combination with in vivo data.
Fluoroquinolones bind to bacterial DNA only during replication. Since the bacterial life cycle consists of periods of quiescence interspersed with periods of active replication, greater-than-MIC fluoroquinolone concentrations must persist over time to catch bacteria when they are replicating and therefore susceptible. Thus, with fluoroquinolones, high drug concentrations with respect to MIC are important, but sustained high concentrations are even more important.
The statistical measure area under the curve (AUC) can illustrate the clinical relevance of high concentrations. For our purposes, AUC describes the concentration of a drug in fluid or tissue over a specified duration from the time of administration. If we overlay the MIC value on the time-versus-concentration curve (Figure 1), we can tell exactly how long the concentration of a specific drug remains greater than the MIC.
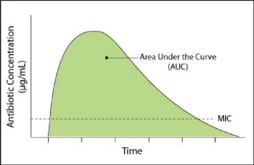
Figure 1. Plotting drug concentration over time and adding MIC value indicates how long greater-than-MIC values persist.
Thus, looking at AUC and MIC together can tell us how long a specific bacterium is exposed to an effective drug concentration at the infection site. This is an index of the drug's ability to achieve bacterial eradication and clinical cure. If the drug were a prize fighter, MIC would be a measure of the fighter's strength and AUC a measure of his stamina.
As an example, Figure 2 plots concentrations over time for levofloxacin 1.5%, moxifloxacin 0.5%, and gatifloxacin 0.3% in rabbit cornea after a single topical dose. In this example, levofloxacin has the highest AUC value.

|

|
Figures 2 & 3. Concentration vs. time curves for levofloxacin, moxifloxacin and gatifloxacin in normal rabbit corneal tissue (left) and aqueous (right) after a single dose.
Levofloxacin Concentrations in Primate Tissues
I have conducted studies of levofloxacin 1.5% in rabbits and Rhesus macaque monkeys. The latter is particularly useful, as monkeys and other non-human primates have eyes much like ours — only primates, for example, have an uninterrupted Bowman's membrane.
In the primate study, normal, healthy Rhesus monkey eyes were randomized to either levofloxacin 1.5% or control (vehicle without levofloxacin) four times per day for 2 days prior to penetrating keratoplasty (PK).1 The animals also received 4 drops at 5-minute intervals in the hour before surgery. Aqueous humor samples were taken by clear corneal puncture immediately before surgery, and the corneal buttons removed by trephination during the PK were cut in half, with one portion sent for histological analysis and the other for determination of drug concentration.
The monkeys received donor corneal buttons and drug dosing continued for a week after PK. Both before and after surgery, confocal microscopy and slit lamp exams were conducted by well-trained masked observers. Neither the histological nor the confocal microscopy studies found any evidence of toxicity in levofloxacin 1.5%-treated corneas versus controls.1
The mean concentration of levofloxacin in the aqueous was 4.49 μg/mL (standard deviation ±1.59); and the mean corneal concentration was 103.85 (standard deviation ±51.8) μg/g, both well above the MIC90 levels for common ocular pathogens (Table 1). While it is necessary to be cautious in extrapolating primate data to humans, these data suggest that topical levofloxacin 1.5% will deliver effective drug concentrations to human eyes.
| Table 1. Levofloxacin MIC90 Levels for Common Ocular Pathogens (μg/mL) |
|---|
| Staphylococcus aureus…………0.75 Staphylococcus epidermidis……0.78 Streptococcus pneumoniae……1.56 Serratia marcesens……………3.13 Propionibacterium acnes………0.75 Mycobacterium fortuitum………1.00 Source for all except M. fortuitum: RW Johnson Pharmaceutical Research Institute. Raritan, NJ. Data on file. M. fortuitum source: Rodriquez JC, et al: Int J Antimicrob Agents 2003;21:585-588. |
Comparative Drug Concentrations
Studies measuring levofloxacin levels in rabbit eyes correlated well with our monkey-eye results. To compare tissue penetration of fluoroquinolones, 21 rabbits (42 eyes) were given one drop of levofloxacin 1.5%, moxifloxacin 0.5%, or gatifloxacin 0.3% bilaterally.2,3 Three rabbits were sacrificed at each of the following post-dosing time points: 10 minutes, 30 minutes, 1 hour, 2 hours, 4 hours, 8, hours, and 12 hours.
Levofloxacin and moxifloxacin showed rapid penetration of corneal tissue; however, moxifloxacin also cleared from the tissues very quickly (Figure 2). The last time point at which there were detectable levels of moxifloxacin in the cornea was 4 hours. Gatifloxacin penetrated corneal tissue more slowly than either levofloxacin or moxifloxacin and cleared more slowly than moxifloxacin but was undetectable after 8 hours. Drug exposure — the AUC — was approximately twofold greater for levofloxacin than for gatifloxacin or moxifloxacin in corneal tissue (Figure 2).
Our rabbit studies also looked at aqueous humor concentrations. Not surprisingly, these followed a course similar to corneal concentrations, with moxifloxacin levels rising quite high but also falling rapidly (Figure 3). At 8 hours, moxifloxacin was detectable in only one sample. Gatifloxacin again reached the aqueous more slowly, but levels stayed higher for a longer period than moxifloxacin. Gatifloxacin, however, was not detectable after 8 hours.
While there was a brief window in which moxifloxacin was at higher concentration that levofloxacin 1.5%, that window was fleeting (Figure 3). With its long half-life, levofloxacin was still present at 12 hours while neither gatifloxacin 0.3% nor moxifloxacin 0.5% was detectable in the aqueous. The AUC value was 2.7 for levofloxacin, 2.0 for moxifloxacin and 1.0 for gatiflox acin.3
Toxicity
The pharmacokinetic evidence is clear and compelling: levofloxacin 1.5% administration results in sustained high tissue concentrations with AUC levels greater than other available fluoroquinolones. In my opinion, this makes it the drug of choice for multiple treatment applications.
But what about toxicity at such highly concentrations? Toxicity is a potential danger in any highly concentrated drug. For example, the use of fortified aminoglycosides is limited by their well-known corneal toxicity. This, however, is not the case with levofloxacin. Repeated studies of levofloxacin in clinical concentrations, including my own work in animal models, have found the levofloxacin molecule to be virtually without corneal toxicity.1,4,5
In safety studies conducted to gain FDA approval, healthy human subjects dosed themselves with levofloxacin 1.5% over 2 week periods. In the first group, 100 people administered 74 doses; in the second, 56 people dosed themselves 25 times per day for a total of 350 doses. There was no epithelial damage and no endothelial or inflammatory changes in these subjects.6 Even with extremely high dosing, corneas stayed healthy and normal.
In a recent study presented at the 2008 ASCRS meeting in Chicago, Abelson and colleagues dosed each of 48 healthy volunteers with a total of 224 drops of levofloxacin over 14 days.7 Specular microscopy and pachymetry were conducted at baseline and on day 21. No significant change was observed in any measured parameter. The bottom line is that the efficacy gained by levofloxacin's high concentration doesn't come at the cost of toxicity. Even when dosed at the levels used to treat the most aggressive corneal infections, levofloxacin 1.5% shows no meaningful toxicity. Rapid epithelial healing in the presence of levofloxacin 1.5% can make using it an appropriate choice in corneal applications where bacterial keratitis becomes a concern due to epithelial defect, abrasion or trauma.
Resistance
Although growth of bacterial resistance to antibiotic therapy and changes in patterns of resistance must be continually monitored, at the moment all the major ocular fluoroquinolones — levofloxacin, moxifloxacin and gatifloxacin — have quite acceptable (and quite similar) resistance patterns. While gatifloxacin, moxifloxacin and levofloxacin can be distinguished from each other in a number of ways, they have virtually identical resistance profiles when tested against important ocular pathogens.
This was recently reaffirmed with publication of the long-term TRUST (Tracking Resistance in the United States Today) data.8 Among resistance-tracking studies, TRUST stands out because of its robust methodology and the number of isolates collected. A subset of the TRUST data, Ocular TRUST, involves only ocular isolates. Mining these data, Asbell and colleagues recently reported that resistance to levofloxacin, moxifloxacin and gatifloxacin among important ocular pathogens, including both methicillin-sensitive organisms and methicillin-resistant Staphylococcus aureus and Staphylococcus epidermis, was virtually identical. In addition, there was less resistance to these fluoroquinolones than to non-fluoroquinolone antibiotics.8 The bottom line is that, except in rare instances, resistance is not a useful criterion in selecting among the three major ocular fluoroquinolones.
Conclusion
Pharmacokinetic data argue strongly for the use of levofloxacin 1.5%, and neither toxicity nor resistance patterns argue against that point.9 With levofloxacin 1.5%, we have a broad-spectrum antibiotic with an excellent susceptibility profile, negligible toxicity, and the ability to provide sustained, effective drug concentrations in tissues of interest. This persistence at greater-than-MIC concentrations means that drug will be available when bacteria begin replication — the only time they are susceptible to fluoroquinolones. This helps ensure levofloxacin's efficacy against a range of ocular pathogens, making it a wise choice whenever a broad-spectrum, topical ocular antibiotic is needed. Not only does the research support the use of levofloxacin 1.5% for corneal applications, it is the only fluoroquinolone indicated for the treatment of bacterial keratitis. OM
References
- Gramates PH, McDonald MB, Salib G, et al. Safety and efficacy of levofloxacin 1.5% eyedrops in nonhuman primates having penetrating keratoplasty: clinical and laboratory findings. J Cataract Refract Surg. 2005;31:1995-1998.
- Schneider S, Bezwada P: Penetration and persistence of levofloxacin, moxifloxacin, and gatifloxacin in corneal tissue. Presented at the 2008 Annual Meeting of the Association for Research in Vision and Ophthalmology, April 27-May 1, 2008, Ft Lauderdale, FL.
- McDonald MB, Bezwada P: Penetration and persistence of levofloxacin, moxifloxacin, and gatifloxacin in corneal tissue. Presented at the 2008 Annual Meeting of the Association for Research in Vision and Ophthalmology. April 27-May 1, 2008, Ft Lauderdale, FL.
- Skelnik DL, Clark LA, Bezwada P. Effect of drug concentration and exposure time of levofloxacin, ofloxacin, ciprofloxacin, gatifloxacin, and moxifloxacin on human corneal endothelial cells and keratocytes. Invest Ophthalmol Vis Sci 2003:44: E-Abstract 4739.
- IQUIX package insert, Santen, Inc.
- Walters TR, Hart W. Tear concentration of 1.5% levofloxacin ophthalmic solution following topical administration in healthy adult volunteers. Invest Ophthalmol Vis Sci. 2003; 44: E-Abstract 4453.
- Abelson MB, Torkildsen G, Shapiro A, Lapsa I. Corneal effects of 1.5% levofloxacin (IQUIX(r)) in humans. Poster presented at ASCRS-ASOA Symposium & Congress. April 4-9, 2008, Chicago, IL.
- Asbell PA, Sahm DF, Ocular TRUST. Longitudinal nationwide antimicrobial susceptibility surveillance in ocular isolates. Results from Ocular TRUST 2. Poster presented at the Association for Research in Vision and Ophthalmology (ARVO), Ft Lauderdale, FL, April 27-May 1, 2008.
- McDonald MB. Research review and update: IQUIX (Levofloxacin 1.5%). Int Ophthalmol Clin.2006;46(4):47-60.








