Learn the Advantages of Spectral Domain OCT
Obtain more precise imaging and relevant data for improved diagnoses and treatment decisions in retinal disease.

By Peter K. Kaiser, MD
Spectral domain optical coherence tomography (SD-OCT) is opening up doors for OCT technology that we never thought possible. It's exciting to learn about its new capabilities. But this new level of OCT technology isn't just an exciting development. It has many practical applications for today's physician.
Gleaning More Information
Time domain OCT provides us with a large amount of data, but spectral domain affords us exponentially more data because of its dramatically decreased acquisition times. With the increased data, you can visualize the entire macula and produce crystal clear 3-D images. Moreover, SD-OCT devices deliver additional information we're just beginning to learn how to use.
If you're hesitant about switching from time domain OCT to SD-OCT, consider the superior results you'll get when imaging certain areas of the retina. With time domain OCT, it's often difficult to identify abnormalities at the vitreomacular interface, such as subtle epiretinal membranes (ERM). Often, a patient will complain of vision problems due to an ERM that time domain OCT can't identify. However, Cirrus HD-OCT, an SD-OCT device, clearly shows the ERM (Figure 1).
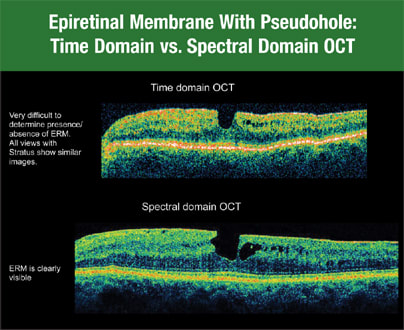
Figure 1. Cirrus HD-OCT clearly shows an epiretinal membrane that time domain OCT may not identify.
SD-OCT is also superior to time domain when evaluating the photoreceptor layer. While you can sometimes see the inner segment-outer segment junction of the photoreceptor layer with time domain, you can see it consistently with SD-OCT. This is important in postoperative patients and those who have unexplained vision loss. For example, when the vision of one of my patients wasn't what I'd hoped for 1 month after macular hole surgery, time domain OCT showed no problems. The hole had completely closed, and everything looked beautiful. However, when I used Cirrus HD-OCT, I saw a small area in 7 the fovea where the inner segment-outer segment junction was still abnormal, and there was a small amount of retinal tissue that needed to coalesce (Figure 2).
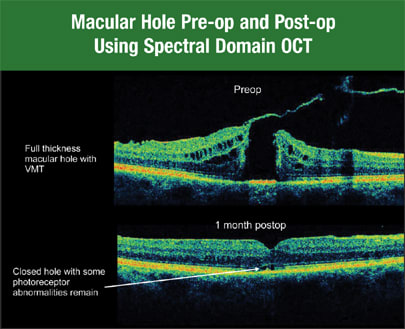
Figure 2. Spectral domain OCT consistently allows you to see the inner segment-outer segment junction of the photoreceptor layer.
Therefore, I was able to tell this patient with confidence that his vision likely would continue to improve as this area healed. Without using Cirrus HD-OCT, I wouldn't have known this, and I would've had to tell the patient, "I really don't think we're going to get additional visual improvement."
I've also found Cirrus HD-OCT to be very useful after retinal detachment repair. When one of my patients complained of poor vision, I needed to know if it would improve. HD-OCT showed a little puddle of fluid that remained in the fovea. Again, I was able to tell the patient, "The fluid is going to resolve, and you'll have better vision. We just have to wait."
3-D Imaging
The single-scan tomogram, however, isn't always the best way to evaluate retinal pathology. Cirrus HD-OCT scans rapidly, gathering a large amount of information we can analyze in new and useful ways. For example, HD-OCT can perform a cube scan that images the entire macula. Often, we can barely see subtle retinal pathology with time domain OCT, but by scanning with a cube scan, we can view pathology easily since we can see it in three dimensions. For example, we can visualize subtle subretinal or intraretinal fluid when treating age-related macular degeneration (AMD) patients with anti-vascular endothelial growth factor drugs. This ability gives us the power to make better treatment decisions for our patients.
If you're looking for vitreous traction, the three-dimensional view allows you to see the relationship between the vitreous and macula, and you can rotate and view it from several angles. As a result, you can more accurately plan your surgical approach if the patient needs surgery.
These 3-D views are also very exciting to show patients. They're the "Gee whiz!" of Cirrus HD-OCT. Single-scan tomograms might give you a 5-line raster through the fovea and a cool view of a macular hole. But if you look at the retina in 3-D and show this to your patients, you can pinpoint the traction descending onto the optic nerve and fovea, and help them understand why treatment options to relieve that traction will work. You also can show the 3-D view to patients to help illustrate macular edema with an ERM. This view is incredibly revealing. Patients get an "Ah-ha!" moment when they see 3-D scans.
Viewing the Layers
The automated segmentation of these scans gives us another opportunity to improve diagnosis and management of retinal disease. For example, we can view the segmented layers of the retina and see how an ERM affects the retinal contours. You can see the crinkling of the internal limiting membrane (ILM). In the future, this might help us decide whether or not a patient needs surgery.
The advanced visualization tool is exciting because we've used the term "optical biopsy" for years with time domain OCT — but now Cirrus HD-OCT technology makes it possible (Figure 3).
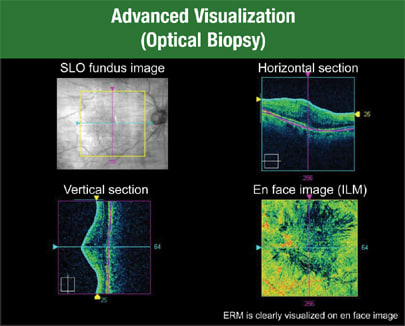
Figure 3. Cirrus HD-OCT allows you to view the ILM map, see it from the top and identify any distortion on the retinal surface. You can guide the view farther down into the retina and examine the choroidal vessels on en-face.
| Evaluating Macular Change |
|---|
Cirrus HD-OCT soon will have a feature called macular change analysis, which calculates changes by comparing images over time. This technology helped me manage a patient with macular edema secondary to branch retinal vein occlusion (BRVO) (Figure 1).
Figure 1. The macular change analysis (available soon) calculates changes by registering and comparing images over time. I gave the patient an off-label intravitreal injection of bevacizumab (Avastin, Genentech), after which she returned with essentially unchanged visual acuity. At first glance, the cube scan of Cirrus HD-OCT didn't look all that different, so I wondered if I should perform another injection. The retinal thickness analysis looked about the same — just a slight change in volume that didn't convince me there was significant improvement. Next, I used the macular change analysis function on the patient's scans. This analysis showed me there had been a dramatic decrease in thickness we didn't appreciate without the change analysis feature. The system registered the images, did a comparison and displayed the change analysis. We decided to treat again based on this analysis. Another patient had choroidal neovascularization due to age-related macular degeneration. I treated the patient with ranibizumab (Lucentis, Genentech), but there was no change in vision. Looking at the B-scans, I'd argue that the OCT images didn't change. So, was the treatment effective for this patient? The retinal thickness analysis looked like the drug therapy worked, but when I performed the macular change analysis, the evidence was even more dramatic. It was easy to see the therapy had been successful. |
In the Cirrus HD-OCT display, I can show the ILM map, view it from the top and see any distortion on the retinal surface. Then I can guide the view farther down into the retina and look at the choroidal vessels. I can see the choroidal vessels on en-face — a view that just isn't available with any other technology to date.
One thing I appreciate about Cirrus HD-OCT is the ability to print all of these images in a way that's both helpful for making diagnoses and meaningful to patients. A standard printout includes everything you'd want to show your patient. New Review software allows us to remotely show these images and videos in our clinic as well.
More Accurate Retinal Maps
Because Cirrus HD-OCT provides so much more information than time domain OCT, we can obtain retinal maps that are considerably more accurate. Time domain OCT captures six radial line scans, and then interpolates between the scans to create a retinal thickness map. HD-OCT gives us a 6-mm cube with almost every point within that grid map measured directly — far more than the area mapped with time domain OCT.
This is very important for a reading center. With time domain OCT, you can miss small lesions that fall between the radial lines, and if there's an error in the segmentation analysis, the software will propagate it into a large area of the resulting retinal thickness map.
In addition, time domain software assumes that all six of the radial lines intersect at one point. In reality, there's eye movement between each of the scans, so they don't intersect at one point. This is a big issue since eye movement can cause the software to artificially under- or overestimate retinal thickness. SD-OCT devices image a larger area of the retina and are much faster, so eye movement becomes less of an issue and we get a more accurate retinal map. This hopefully will translate into more accurate outcomes in clinical trials and when we follow up with patients to determine treatment response.
What's more, the segmentation algorithms of Cirrus HD-OCT have improved its ability to identify the retinal boundaries in patients with AMD and choroidal neovascularization. Retinal thickness measurements in patients with AMD can be inaccurate with time domain OCT. In contrast, HD-OCT mapping is extremely precise. Moreover, if the lines don't accurately reflect the location of the inner and outer retina, we can move the lines to correct the issue. This isn't possible with time domain devices.
Finally, Cirrus HD-OCT offers dramatically improved registration between visits. We can register a patient's retinal maps to ensure we're imaging the same place every time we perform our scans. We can compare certain locations on the retina over time. Therefore, we'll know if the patient's vision has changed as a result of disease progression or clinical treatment. Registration accuracy at this level simply isn't possible with time domain OCT.
Dramatic Shift
Cirrus HD-OCT has dramatically changed the way I practice, and I think it will change the way we conduct clinical trials. The accuracy of the boundary detection has improved significantly, giving us more precise and more relevant measurements for both clinical practice and clinical trials. Scanning speed has become so amazingly fast that we can attain highly detailed cubes of data for 3-D analysis, and we can examine a large area of the macula. Hopefully, this will improve our diagnosis and treatment decisions in the future. OM
Dr. Kaiser is director of the Digital OCT Reading Center of Cole Eye Institute at the Cleveland Clinic Foundation. He's study chairman of the DENALI trial, and principal investigator in multiple national, multicenter clinical trials.








