At Press Time
Posting Eye Surgeries on YouTube
A New Vehicle for Education, Information Exchange.
By Leslie Goldberg, Associate Editor
■ YouTube was an instant hit when it launched in February 2005 as a way for tech-savvy individuals to share homemade video clips with their friends, and the public at large, via the Internet. The site's 4-year history has been marked by steady evolution in both its technical capabilities and the respectability of its offerings, thanks in no small part to its acquisition by Google in November 2006.
Increasingly, it's being embraced by professionals as well, and the abundance of YouTube's ophthalmic offerings indicate that ophthalmologists are no exception.
While it is important to note that you should look for credible doctors' names and be cautious when viewing videos posted on YouTube, it is a resource with many benefits. The ability to post vast amounts of information in a video format has many uses for ophthalmologists — patient education, international exchange of ideas and introduction to new procedures, to name a few.
Having instant access to surgical videos, particularly ones that are instructional and well edited, is helpful for the ophthalmic community, according to Uday Devgan, M.D., F.A.C.S., a partner at the Maloney Vision Institute in Los Angeles, chief of Ophthalmology at Olive View UCLA Medical Center and associate clinical professor at the UCLA School of Medicine.
"As a resident 10 years ago, I used to hunt down copies of Bob Osher's video journal of cataract and refractive surgery to learn things such as Nagahara's Phaco Chop technique," says Dr. Devgan. "Now it's much easier. I have instant access via the Internet to dozens of videos of the same technique, and thousands of total eye surgical videos."
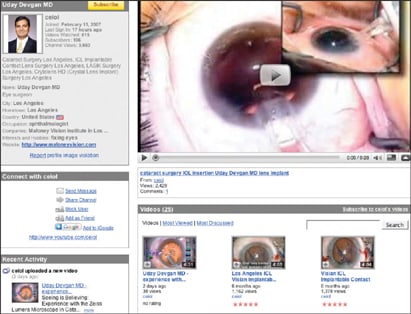
Dr. Devgan's YouTube page lists the videos he has contributed to the site. Image courtesy of Uday Devgan, M.D.
Dr. Devgan, who spends 1 day a week teaching UCLA residents, says they do a significant amount of learning via video before coming to the operating room. "A few years ago, I would burn a DVD with about 50 of my videos and hand them out. Now I simply tell them to go to my teaching website (www.UdayDevgan.com) to get links to watch videos, download videos, download talks, etc."
"I have posted the DSEK Suture Pull Technique on YouTube as a way to make the technique available to other ophthalmologists outside of the national meetings," says Andrew Sorenson, M.D., a partner at Sorenson Eye Associates in Berkeley, Calif. "This procedure is rapidly evolving and the access to various techniques and outcome studies has helped me immensely. I initially hoped the video would assist others. The feedback I have received suggests this has been the case."
In addition, Dr. Sorenson teaches at the Alameda County Medical Center for the California Pacific Medical Center Department of Ophthalmology. He says that the videos on YouTube have assisted the residents there in preparing for surgical procedures as they have attempted to understand the varying preferences of the attending surgeons who oversee their work.
Ignacio Manzitti, M.D., of Buenos Aires, Argentina says that the primary reason he posts video on YouTube is so he can tell his patients that if they want to view a particular surgery he has recommended, they can go online, search his name on YouTube and watch a surgery that has been performed by him. Dr. Manzitti says patients greatly appreciate this and watching the videos at home with their family makes them more relaxed when they come to the OR.
"We use telemedicine to do rounds, including videos," assertd Dr. Manzitti. "I think this is a new instrument to educate. It helps us to better communicate what we do."
| IN THE NEWS |
|---|
| ■ Aquashunt trial begins. OPKO Health, Inc. said it has begun treating patients in a clinical trial of its Aquashunt, a device for the treatment of refractory open-angle glaucoma. The study, being conducted in the Dominican Republic, will assess the safety and efficacy of the Aquashunt and enroll up to 20 patients with significantly impaired visual acuity. The Aquashunt is designed to lower IOP by allowing excess fluid in the eye to exit more naturally than occurs with presently available devices. "We are pleased by the simplicity of the surgical procedure for the device and by the early results in the first human trials," said M. Bruce Shields, M.D., chairman emeritus of ophthalmology at Yale University and designer of the Aquashunt. ■ Pfizer to acquire Wyeth. Pharmaceutical giant Pfizer, which produces a leading glaucoma medication, Xalatan, has agreed to acquire rival Wyeth in a cash-and-stock deal valued at approximately $68 billion. The boards of directors of both companies have approved the combination. Until 2001, Wyeth manufactured the widely used ophthalmic spreading agent Wydase. However, Wyeth discontinued production of Wydase when the manufacturing facility for that compound was closed, causing ophthalmic surgeons to seek alternative sources of spreading agents. Wyeth has not had a significant presence in ophthalmic products since 2001 but the company is involved in ongoing research for potential treatments for retinal diseases. Pfizer has a research partnership with Quark Pharmaceuticals designed to develop treatments for retinal diseases. Pfizer also announced that U.S. sales of Xalatan climbed approximately 2% in 2008 to $144 million. ■ Restasis sales soar. Allergan reported that total sales of Restasis, the company's prescription treatment for dry eye, jumped to $444 million in 2008 from $344.5 million in 2007, a year-over-year increase of 28.9%. The company also projected 2009 Restasis sales in a range from $490 million to $510 million. Allergan's glaucoma medication Lumigan recorded an 8.8% year-over-year increase in sales to $426.2 million, while combined sales of the glaucoma medications Alphagan, Alphagan P and Combigan jumped 16.6% to $398.1 million. ■ Alcon toric lens approved. Alcon's AcrySof IQ Toric IOL has been approved by the FDA. The AcrySof IQ Toric lens combines aspheric optics found in the AcrySof IQ and the astigmatic correction of the existing AcrySof Toric lens. In related news, Alcon said its glaucoma medications and IOLs experienced double-digit sales growth in 2008. ■ Sirion NDA approved. Sirion Therapeutics, Inc. said that its New Drug Application for ganciclovir ophthalmic gel 0.15% has been accepted for review by the FDA. Sirion is seeking approval for ganciclovir as a treatment for herpetic keratitis, an ocular disease caused by the herpes simplex virus. The FDA has issued an action date in late fall of 2009, under the Prescription Drug User Fee Act. ■ Alcon a "Best Workplace." For the 11th consecutive year, Alcon's U.S. affiliate, Alcon Laboratories, Inc., was named to Fortune magazine's "100 Best Companies to Work For" list, placing 74th. ■ Ophthalmic technicians recognized. The Joint Commission on Allied Health Personnel in Ophthalmology (JCAHPO) said that the ophthalmic allied health profession has received official notification of approval for a separate occupational classification, Ophthalmic Medical Technician, from the United States Bureau of Labor's 2010 Standard Occupational Classification (SOC) Committee. "This is a historic milestone for the ophthalmic allied health profession," said JCAHPO President William F. Astle, M.D., FRCS(C). "Ophthalmic Medical Technicians are important in the eyecare team as ophthalmology becomes more technologically oriented, and confronts the challenges of an aging patient population. This is a significant measure of how much this profession has evolved into its own, and an empowering validation of this occupation's professional merit." The 2010 SOC system is used by federal statistical agencies to classify workers into occupational categories for the purpose of collecting, calculating and disseminating data. For an occupation to be accepted for inclusion in the SOC, it requires a set of uniquely identifiable skills. The first set of skills is related to the complexity and range of tasks and duties, including knowledge and experience. These are defined by preparation levels and credentials, and considered necessary for new entrants to an occupation. The second set is related to both the type of work performed and the nature of the work activities. ■ Inspire drug in new trial. Inspire Pharmaceuticals, Inc. has reached agreement with the FDA through a Special Protocol Assessment on the design of a new phase 3,450-patient clinical trial for Prolacria (diquafosol tetrasodium ophthalmic solution 2%) for the treatment of dry eye disease and has recently initiated enrollment in the trial. The drug approval process for Prolacria has been stalled since 2006, with the FDA requesting an additional confirmatory study after issuing two "approvable" letters to Inspire. The FDA indicated, as part of the review process, that even if this trial is successful, the FDA's review of Prolacria will also take into account the robustness of the trial results, that a surrogate endpoint was used, the results from previous Prolacria trials and the overall risk/benefit. OM |








