IOLs: State of the U.S. Market
A variety of new options means better vision for patients.
BY REN� LUTHE, SENIOR ASSOCIATE EDITOR
With the cataract surgery market expected to enjoy healthy growth as the Baby Boomer generation enters its golden years, IOL makers have been busy improving lens technology. “Global population growth and demographic shifts, combined with the patient’s willingness to pay for the best visual outcome, will continue to drive demand for better vision technologies,” explains Jim Mazzo, chairman, president and chief executive officer of Advanced Medical Optics’ (AMO, Santa Ana, Calif.). Thus manufacturers continue their quest to fix presbyopia, whether through accommodating or monofocal IOLs, while also battling the aging process with aspheric lenses to improve contrast sensitivity. The result of all this innovation is more possibilities to give today’s cataract patients the quality of vision they are increasingly demanding. Here, industry leaders and cataract experts tell us how the IOL market is evolving.
PC-IOLs Continue to Gain Ground
Delivering presbyopia-free vision after cataract surgery continues to be the optical Holy Grail. And the 2005 CMS ruling on “patient-share” billing for presbyopia-correcting IOLs (PC-IOLs) has made this technology more affordable for patients, providing a significant boost to their sales. Uday Devgan, M.D., chief of ophthalmology at Olive View-UCLA Medical Center and assistant clinical professor at Jules Stein Eye Institute, Los Angeles, calls PC-IOLs “the real deal … they certainly have the ability to provide a great deal of spectacle independence and convenience for the right patients.” According to a recent MarketScope report, approximately 125,000 PC-IOLs were sold in 2006. Here is a list of PCIOLs currently available on the U.S. market.
• Accommodative IOLs. Currently there is only one accommodative IOL available on the U.S. market, the crystalens (eyeonics, Aliso Viejo, Calif.). It’s now the crystalens 5-0, an improvement, the company explains, that features a 5.0-mm diameter optic (vs. the former 4.5-mm optic) and delivers even less incidence of glare and halo. Additionally, the crystalens 5-0, which was launched in November 2006, features a 360º square edge to eliminate CCS, 17% greater surface area contact between the optic plate and capsular bag, 90% more plate arc length for more capsular bag support and parallel plates (vs. trapezoidal plates). The lens is also now available in an expanded diopter range, eyeonics says. The improvements seem to have made a hit with surgeons, according to the company — it reported $5.5 million in revenues for the first quarter of 2007, a 35% increase from the year-ago quarter.
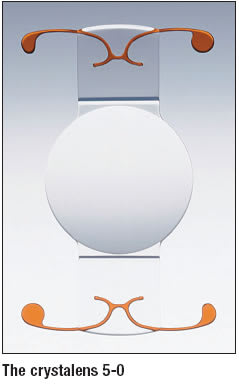
“I have started to implant the crystalens and have found it to be a wonderful lens that has lived up to its claims,” says Michael S. Korenfeld, M.D., of Washington, Mo. “I still have limited experience with it, but there are no complaints of glare and halos with this lens.” Eyeonics should not have the accommodative niche all to itself much longer, however; Visiogen’s (Irvine, Calif.) Synchrony dual-optic accommodating IOL is currently in phase 3 clinical trials. Company President and Chief Executive Officer Reza Zadno, Ph.D., says Visiogen anticipates FDA approval in 2009. The lens, with proprietary preloaded injector, has the potential of providing greater accommodation because of the high-power moving lens. The single-piece silicone IOL unfolds upon insertion and features two optics connected by a spring system. The springs connect a 5.5-mm anterior optic and a 6-mm negative-power optic, while the spring action moves the front optic and changes the eye’s focus from near to far.
Last year, Visiogen obtained Europe’s CE Mark for the Synchrony. The company is now pursuing post-marketing research studies in Europe. The Synchrony should enter markets there in 2008, Dr. Zadno says. Meanwhile, Lenstec’s (St. Petersburg, Fla.) Tetraflex accommodative IOL is also in phase 3 trials. The company anticipates a 2008 launch for the lens, which is already available in Europe. The Tetraflex is an acrylic IOL with a 5.75-mm optic; its haptics are designed with a “contoured effect” that allows flexibility within the accommodation process so the lens can move anteriorly in the capsular bag secondary to ciliary muscle contraction. Lenstec offers the IOL in quarter-diopter increments to increase predictability in cataract surgery.
• Multifocal IOLs. Alcon’s (Fort Worth, Texas) AcrySof ReSTOR apodized diffractive IOL has been implanted in nearly a quarter of a million eyes globally since its release in 2005 and MarketScope cites that the AcrySof ReSTOR is the most frequently implanted PC-IOL in the U.S. Market. AMO’s ReZoom multifocal IOL is a refractive zonal lens, offering five focusing zones for what the company says is a full range of vision. AMO says the lens’s “competitive strength” is its distance and intermediate vision. AMO has another multifocal in the pipeline — Tecnis Multifocal. The company describes it as a full diffractive refractive IOL with a proprietary aspheric surface that is based on Tecnis technology. The result is that it offers the contrast sensitivity benefits of the Tecnis Aspheric lens, which reduces spherical aberration and improves vision in lowlight conditions. It has already received regulatory approval in Europe and some other international markets. While the swelling ranks of presbyopic cataract patients seem to mean good things for the multifocal IOL market, some surgeons caution that the lenses haven’t quite made the grade yet. “The multifocal and accommodating lens implants are more popular than a year ago, but I don’t think anyone believes they have ‘arrived,’” says Paul Koch, M.D., medical director of Koch Eye Associates, Warwick R.I., and Opthalmology Management’s chief medical editor.
“As long as manufacturers have to provide seminars on how to sell the lens, they will not be considered mainstream. When patients actively seek them out — not when surgeons introduce and sell them — that’s when we say they’ve arrived.” Even Dr. Devgan cautions that in order for multifocals to “work” for patients, surgeons must carefully review with them their visual needs. “I find that the majority of my exam time with patients is spent educating them, learning about their visual needs and desires, and helping them to select a technology that will meet or exceed their expectations,” he says. One of his favorite clinical pearls: “Underpromise and overdeliver.” In addition to questions about the patient’s daily activities and driving and computer habits, he recommends asking what patients would like to change about their eyes. “What would you like to accomplish with this eye surgery?” For all the care in patient selection, Dr. Devgan believes PC-IOLs are bound to gain an increasingly larger market share.
“They already account for more than half the IOLs I implant today,” he says. “That trend will only increase as the technology gets better.” Such comments may be the reason manufacturers are creating “new and improved” multifocals. In addition to AMO’s Tecnis Multifocal that is on the way, Alcon reports that the FDA recently approved the addition of an aspheric component to the ReSTOR that is designed to improve image quality. The company also plans to submit a lowpower ReSTOR IOL for FDA approval later this year.

Aspherics Lead Monofocal Pack
For all the buzz about PC-IOLs, aspheric monofocals continue to play a crucial role in the U.S. IOL market, according to both manufacturers and surgeons. Recent New Technology IOL (NTIOL) designations have contributed to their popularity. Christine Oliver, Bausch & Lomb’s vice president of professional communication, surgical products, says that the aspheric-technology IOL is the fastest-growing monofocal lens and will continue to enjoy rapid growth due to the preservation of contrast sensitivity it offers as opposed to other IOLs. “Most cataract patients are over 65, most in their 70s,” Oliver points out. “As we age, naturally we lose some contrast sensitivity, especially in low light, so compromising that [with an IOL] can be potentially unsafe for older patients.” According to Dr. Devgan, aspheric IOLs should account for 95% of the monofocal IOLs that are implanted. He points out that newer multifocal IOLs will incorporate aspheric optics to optimize image quality. “Having an IOL (of any type) with aspheric optics will be standard in the near future,” Dr. Devgan predicts, “the same way that nearly all IOLs have UV coatings (to protect the retina) and squared edges (to prevent posterior capsule opacification).”

“Aspherics are really wonderful and I believe in them,” concurs Dr. Korenfeld. “I think it would be best to choose an aspheric monofocal for all patients who are not interested in an accommodative IOL or who need a toric lens.” STAAR Surgical apparently agrees; the company says that both its best-selling three-piece Collamer and three-piece silicone IOLs will feature an aspheric surface for improved visual quality. Both are expected to launch this summer.
Currently available to U.S. surgeons and their patients are B&L’s Sofport AO, Alcon’s AcrySof IQ and AMO’s Tecnis. All three manufacturers report seeing a significant increase in sales since gaining NTIOL status. In fact, Alcon claims that its 2006 domestic unit sales for its AcrySof IQ lens were 98% higher than they were for 2005. The primary difference between the three aspherics on the U.S. market is aspherical correction factor. The AMO’s Tecnis and Alcon’s AcrySof IQ feature negative spherical aberration algorithms; The Tecnis’s is -0.27 and the AcrySof IQ’s is -0.17. B&L’s SofPort AO is an “aberration-free aspheric,” according to Oliver. Both negative-aberration designs operate on the principle of counteracting the mean amount of spherical aberration found in the general population of cataract patients so that the resulting total spherical aberration of the eye is zero.
Whereas “the SofPort AO has no built-in prejudice, if you will,” Oliver says. “it’s totally aberrationfree across the entire lens surface. The reason we believe that is very important is that as the healing process occurs and the bag shrinks around the IOL, in certain lenses, the shrinkage causes the lens to tilt or rotate. We believe that makes changes as to where the lens functions relative to the optic nerve.”

Addressing Astigmatism
According to a 2006 MarketScope report, nearly 40% of cataract patients have astigmatism. In seeking to deliver to cataract patients the superior vision they want, manufacturers are creating lenses designed to correct this refractive disorder. Alcon released the AcrySof Toric in 2006. The company reports that sales were limited due to the lack of CMS coverage.

However, since the CMS ruling that allows Medicare beneficiaries access to astigmatism-correcting IOLs, Alcon says there has been a “dramatic increase” in the number of physicians implanting the lens. STAAR’s Toric IOL was introduced to the U.S. market in 1998.
Light-filtering Technology
While all IOLs have some UV protection, an increasingly popular addition to the IOL market are lenses that aim to protect the retina from potentially hazardous light. Alcon’s blue-light filtering AcrySof Natural was approved by the FDA in 2003. A 2006 MarketScope report indicates that while approximately two-thirds of cataract surgeons were not sure that blue-light filtering really benefited the retina, the AcrySof Natural and IQ lenses accounted for nearly one-third of the IOLs sold domestically. Studies have been performed that demonstrate blue-light toxicity and that blue-light filtering may provide retinal protection.
Last year, B&L joined the light-filtering cause with its Sofport AO with Violet Shield. As its name suggests, “The lens provides additional high-energy light protection for the retina without affeccting color perception or contrast sensitivity,” says Oliver. “Studies have demonstrated that high-energy violet light can be toxic to the retina.” She reports that the lens is being well-received by both current SofPort users and non-users.
The Shape of Things to Come
With demographic trends so promising for the IOL industry, don’t expect innovation to slow down soon. Here is what industry leaders see in the future: Alcon says that given the recent CMS rulings on billsharing for PC-IOLs, NTIOL designations and giving Medicare patients access to astigmatism-correcting IOLs, cataract patients have many options to consider. “By having access to a broader spectrum of IOL options, physicians and their staffs will spend more time with patients to fully understand the patient’s lifestyle, visual needs and to set postop expectations,” an Alcon spokesperson says. All manufacturers anticipate that the PC-IOL segment will continue to grow.
Toward that end, eyeonics says it will launch a new lens that will provide optimum corneal shape for cataract and presbyopia correction over the next year. AMO’s Jim Mazzo predicts that “mixing and matching” IOLs will continue to increase in popularity. B&L’s Oliver notes a growing interest in and adoption of sub-2–mm incision cataract surgery techniques. She says this trend will only increase as micro-incision IOLs and inserters (for in-the-bag surgery) become available. In anticipation, B&L has submitted its Akreos acrylic IOL to the FDA review process. The foldable IOL offers aberrationfree optics and the potential to be used for micro-incision implantation. Oliver also predicts that more stable nextgeneration torics are part of the future for U.S. markets. These new and impending innovations in IOL technology means that surgeons will be able to deliver to the exacting baby boomers the quality of vision they expect.








