lenses
New Implantable Lens Holds Promise
The Visian ICL greatly expands refractive
options.
DAVID C. BROWN,
M.D., F.A.C.S.
Dioptric |
Overall |
Optic |
Power (D) |
Diameter (mm) |
Diameter (mm) |
-3.0 to –16.0 D |
12.1 |
4.9 - 5.8 |
-3.0 to –16.0 D |
12.6 |
4.9 - 5.8 |
-3.0 to –16.0 D |
13.2 |
4.9 - 5.8 |
-3.0 to –16.0 D |
13.7 |
4.9 - 5.8 |
| Figure 1. Power and Sizes Chart | ||
The recent FDA approval of the Visian (STAAR Surgical, Monrovia, Calif.) implantable collamer lens (ICL) is an opportunity to revolutionize the care of refractive patients. Currently, the Visian ICL is available for the treatment of myopia (Figure 1) and offers advances and options in patient care and a tremendous boost in the refractive surgery arsenal. Advantages of the Visian over laser-based procedures include superior visual results, faster recovery, correction stability and patient comfort, as well as the potential option of reversibility. Avoiding laser procedures eliminates the need for retreatment and the possibility of flap complications or sight-threatening ectasia.
From patient care and economic standpoints, the surgeon is also rewarded in that the Visian ICL implant procedure can be performed in a typically equipped ambulatory surgery center (ASC), eliminating the need for the capital equipment infusion required by laser treatments and the need for enhancements, upgrades and "click fees."
Once it receives FDA approval, the toric version of the myopic Visian ICL will further expand the IOL's indications. STAAR has submitted the clinical study data and awaits the FDA's decision, which could come as early as this month.
|
|
|
Figure 2. Iridotomies patent (seen after implantation) |
Positioning the Visian ICL in a practice that offers refractive lens exchange, multifocal lenses, accommodative lenses, blended vision and wavefront-guided laser procedures can become a mental, and management, juggling act. Training of surgeons and staff to sort out the many refractive options and present them in a logical manner to prospective patients is essential and extremely challenging.
The easiest decision is when the only option is a phakic IOL, as with young patients who are not suitable for LASIK. This includes individuals who have thin corneas or keratoconus or are out of range for laser treatment. More challenging are patients who might be candidates for a phakic IOL, LASIK or clear lens replacement. Most people are relatively well informed about LASIK, and most patients know someone who has had LASIK with a great result. Thus, conversion of a patient from LASIK to a phakic IOL may be more difficult because of the Visian's newness and unfamiliarity. Conversion attempts can result in patients becoming confused and electing to not proceed with their opportunities for improved eyesight by any suitable option.
Introducing a totally new concept such as the Visian ICL requires time, effort and commitment. Important interactions occur in the office between the surgeon and staff, who will offer information and enthusiasm to the patient. Medical announcements on TV and radio are useful, as are limited printed announcements, which help bring the Visian onto the "radar screen." Seminars or previous Visian patients can also be informative or provide testimony on successful procedures, as well as using the Visian Web site and your own practice's Web site.
|
|
|
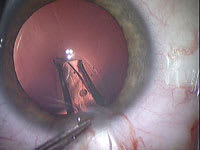
Figure 3. Corneal incision, 2.5 mm parallel to iris plane |
Getting Started
Surgeons' first requirement is to attend a didactic course sponsored by STAAR. They must then schedule five proctor-supervised Visian ICL implantations to be implemented on-site in the their ORs. These milestones have been successfully developed by eyeonics (Alisa Viejo, Calif.), Alcon (Fort Worth, Texas) and Advanced Medical Optics (Santa Ana, Calif.) and are reasonable ways to assure the best outcomes for the rollout of new IOLs.
As with other refractive lens surgeries, absolute accuracy in biometry is required to achieve the expected stellar outcomes. Another requirement is correct sizing of the Visian diameter to make sure that the vault of the lens in the posterior chamber is appropriate. The diameters are optimized by the Visian program that appears when the lens is ordered online from STAAR. Our white-to-white measurements are obtained with the IOL Master (Carl Zeiss Meditec, Dublin, Calif.) and Orb Scan (Bausch & Lomb, Rochester, N.Y.). An additional white-to-white on the OR stretcher with a caliper and ruler can confirm the preoperative measurements. The anterior chamber depth must be more than3 mm, and precautionary endothelial cell counts both pre- and postoperatively are required by the FDA.
|
|
|
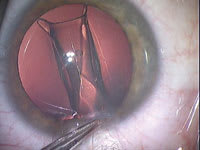
Figure 4. Lens injection with MicroSTAAR injector |
Preparation
Before Visian implantation, Nd:YAG laser peripheral iridotomies (LPI) are performed (Figure 2). The iridotomies must be through the iris stroma and pigment and placed at the 11 o'clock and 1 o'clock positions so the IOL will not occlude the iridotomy. The iridotomy size should be approximately 0.8 mm. If there is doubt about the adequacy of LPI, a third or fourth iridotomy can be made. Nepafenac (Nevanac, Alcon) b.i.d. for 3 days is recommended following LPI.
The patients are placed on moxifloxacin (Vigamox, Alcon) t.i.d. beginning 2 days before their implantation. Postop care requires moxifloxacin t.i.d. and prednisolone t.i.d. for a week. Drops are then discontinued, and follow-up visions and tensions are scheduled for 1 month postop.
Surgical Technique
Sideport incisions of 1.4 mm are made in clear cornea at 12 o'clock and 6 o'clock with a 1.4-mm diamond Brown Universal Cataract Knife (BUCK, Diamatrix, The Woodlands, Texas). Dextran (Ocucoat, B&L) is instilled in one of the sideport incisions, deepening the anteriorchamber. A 2.5-mm diamond keratome incision is placed in clear cornea temporally (Figure 3). It is very important to avoid incising the crystalline lens, so the incisions should be parallel to the plane of the iris. As with phaco flip, the dextran is injected not only to displace aqueous humor but to keep the chamber deep. However, dextran is not used under the iris. The Visian is injected through the 2.5-mm incision and slowly unfolded in the anterior chamber. The Visian is best bumped or nudged into the eye through the injector because steady pressure is more apt to twist or invert the lens. Care is taken to see that the orientation mark is upper right and bottom left on the lens haptics as the lens unfolds. When slowly injecting the IOL, the cartridge and lens are directed posteriorly so the IOL does not float up against the cornea (Figures 4 and 5). Should the lens become inverted, the corneal incision is enlarged, the chamber deepened with dextran and the lens removed. It can then be implanted and folded with forceps after inspection and reorientation.
|
|
|
Figure 5. ICL slowly unfolds, while lens position is monitored |
The lens haptics are positioned under the iris by sliding the Visian radially with no downward pressure on the patient's crystalline lens (Figure 6). The nasal or temporal haptics can be positioned in either order, depending on the surgeon's choice. There are several instruments available for tucking the Visian. We currently use the tip of the dextran cannula or a Batlle ICL Manipulator spatula (Rhein Medical, Tampa, Fla.).
The myopic Visian optic is very thin centrally, so absolutely no touch is permitted on the optic, thus avoiding trauma to the crystalline lens through the optic. All positioning of the Visian is via haptic management.
STAAR has developed a lens-loading procedure that incorporates its MICROSTAAR Injection System and a microforceps instrument developed by MicroSurgical Technology (Redmond, Wash.) that helps position the Visian ICL in the cartridge. The lens is then easily injected with the proper anterior-posterior orientation, since positioning of the Visian is essentially guaranteed by the cartridge and gentle intermittent advancement of the plunger, greatly reducing the chance of a malpositioned lens.
Following positioning of the Visian ICL, the dextran is irrigated from the anterior chamber by a combination of irrigating and aspirating cannulas, similar to the bimanual cataract extraction using irrigation and aspiration or by gentle lavaging with balanced saline solution on a 5-mL syringe. Once the dextran is evacuated from the eye, acetylcholine (Miochol, Novartis) is instilled. As the pupil contracts, another visual check of each laser iridotomy is made to be sure they are patent. In most cases, a small stream of acetylcholine is run through the iridotomy to be sure that it is open and not blocked by the dextran.
The Visian ICL procedure does not require any significant change in the ASC environment. However, efficiency of this system is important for patient comfort and safety. All supplies and instruments must be carefully prepared before implantation. Implantations can be done on one eye or both eyes during the same day. In all cases of bilateral implantations at our facility, the first eye is implanted and the patient is then returned to the preop area, and then the second eye is treated with dilating drops and anesthetized. Meanwhile, complete changeover of the OR is performed, including selection of different lots for supplies and solutions. The instruments are resterilized, the patient is repositioned, prepped and draped, and the second surgery begins.
|
|
|
Figure 6. Lens tucked after verifying orientation |
Though the Visian technique does not require significant changes for an intraocular surgical procedure, it is wise to remember we are dealing with a healthy but potentially "unhealthy" eye with regard to high degrees of ametropia. To ensure a flawless procedure, the following steps should be performed. Most of these steps are obvious.
Before entering the OR:
1. Visian ICL and backup are available
and correct power and size are verified
2. Pupil is well dilated and patient
is relaxed
3. Good anesthesia, topical or injection
is used
4. Iridotomies confirmed to be patent
Before entering the eye:
1. Do "time out" check
2. Room setup and draping complete;
sterile handles on scope
3. Disposables opened and ready on
the table
4. Irrigation and aspiration (I&A)
set up and tested functional (if used)
5. Visian ICL loaded in injector,
ready for implantation (reconfirm power, size and patent)
6. Second Visian ICL available in room
for backup
7. Inject and position Visian ICL,
noting orientation
8. Means to enlarge corneal incision
9. Suture set with 10-0 nylon ready
in room
10. Remove dextran
and constrict pupil with acetylcholine
11. Verify lens position
and wound integrity; inspectiridotomies
Postop Procedures
Relaxing incisions may be performed with the Visian ICL to address astigmatism. The limbal relaxing incision (LRI) procedures are based on the Nichamin nomogram and have provided a reliable way of achieving excellent UCVA combined with the spherical Visian ICL correction.
Additionally, patients who are outside of the range of the Visian ICL can be treated with an excimer laser after a short stabilization period. The LASIK procedure is not complicated by the presence of a phakic IOL, and touchup power changes are generally of a relatively minor residual refractive error and are easily accomplished.
While the patient is still on the table, moxifloxacin (Vigamox, Alcon) and brimonidine (Alphagan P, Allergan) drops are instilled in the operative eye. The patient should be brought to the recovery area for discharge or to prepare for consecutive surgery. After discharge, the surgeon should measure IOP 1 mm Hg to 2 mm Hg and vision. No patch or shield is required.
On the first postop day, expect good vision. The surgeon should check refraction, anterior chamber depth, pupil size, vault and medication compliance. On day 7, the exam should be repeated and drops discontinued. Normal activity may be resumed. A month postop, the surgeon should schedule Vision/Applanation tension and lens position and vault. A routine eye exam should following in 6 to 12 months, including endothelial cell counts.
David C. Brown, M.D., F.A.C.S., the medical director and founder of Eye Centers of Florida in Fort Myers. He can be reached at (800) 226-3377