at press time
Addition Acquires AlphaCor Implants
Artificial Cornea Expands Line of Therapeutic
Devices.
Addition Technology, Inc., of Des Plaines, Ill., maker of Intacs corneal implants for keratoconus and myopia, has acquired AlphaCor and AlphaSphere from CooperVision Surgical. AlphaCor is a flexible, biointegratable, one-piece artificial cornea (keratoprosthesis) designed to replace scarred or diseased native corneas. AlphaSphere is a soft, biocompatible orbital implant that the company plans to launch later this year.
"Similar to Intacs implants, AlphaCor and AlphaSphere are innovative specialty therapeutic devices that address acute conditions of the eye. AlphaCor immediately allows us to capitalize on our relationships with corneal surgeons already using Intacs implants to treat keratoconus," says William M. Flynn, Addition Technology president and chief executive officer.
|
|
|
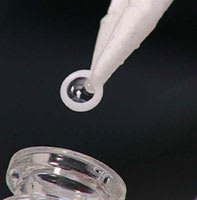
The AlphaCor artificial cornea. |
The World Health Organization reports that corneal blindness affects more than 10 million people worldwide, but only 100,000 receive corneal transplants each year. This shortfall is due to a combination of inadequate supply of donor corneas and the unsuitability of some patients to receive a corneal graft.
AlphaCor is designed for use in patients who have had multiple failed corneal transplants or in those patients for whom a donor graft is likely to fail. Its unique material and patented design features promote retention of the device, minimize postoperative complications and restore vision in patients who cannot receive, or are unlikely to have a beneficial outcome from a human donor graft. AlphaSphere is approved by the FDA for treatment of patients who have lost an eye due to disease or trauma.
Addition Technology, Inc. has also reported that announced second quarter 2006 sales of Intacs increased by 55% vs. the same quarter in the prior year and 20% over first quarter 2006. This marks the company's thirteenth consecutive quarter of double-digit growth in the U.S. and international markets. Growth was driven by increased success of obtaining insurance reimbursement for the Intacs procedure. To assist with the insurance reimbursement process, Addition Technology has established www.Intacsreimbursement.com as well as direct assistance at 847-297-8419 and intacs@intacs-ati.com.
"With any new technology, the adoption rate builds based upon successful patient outcomes experienced by surgeons. Clinical successes and more consistent medical insurance reimbursement have driven growth in the United States," says Flynn. "Internationally, we have a strong, technically oriented distributor network that continues to aggressively train and assist a broad group of corneal surgeons."
STOCK
WATCH A LOOK AT THE PERFORMANCE OF OPHTHALMIC COMPANIES 6/15 6/15 EYE $41.84 32.04 49.29 JNJ 64.58 56.65 65.38 ACL 111.12 77.66 148.70 LCAV 34.60 29.76 58.25 AGN 114.85 69.01 117.78 LUME 1.40 1.25 2.90 BOL 51.14 40.75 87.89 MDT 48.62 42.37 59.87 BDX 73.58 49.71 74.25 MRK 43.20 26.13 43.54 COO 56.00 43.99 78.50 NVS 57.09 45.36 58.57 ESMC 3.35 3.30 6.20 RHEO 2.40 1.56 12.85 DNA 82.74 43.90 100.20 OSIP 36.86 20.81 38.50 HTI 2.75 1.50 3.50 PFE 27.59 20.27 28.51 ISPH 5.40 4.52 16.81 QLTI 7.88 5.97 17.30 ILSE 18.72 12.26 24.38 STAA 7.77 3.12 9.53 IRIX 9.28 3.65 13.40 SURG 5.53 1.40 8.00 ISTA 5.86 5.26 11.24 TLCV 4.45 4.01 8.00
COMPANY
SYMBOL
CLOSE
52-WEEK
LOW
52-WEEK
HIGH
COMPANY
SYMBOL
CLOSE
52-WEEK
LOW
52-WEEK
HIGH
Advanced Medical Optics
Johnson & Johnson
Alcon
LCA-Vision
Allergan
Lumenis
Bausch & Lomb
Medtronic
Becton Dickinson
Merck & Co.
The Cooper Companies Inc.
Novartis
Escalon Medical
Corporation
Occulogix
Genentech
OSI Pharmaceuticals
Halozyme Therapeutic
Pfizer
Inspire Pharmaceuticals
QLT, Inc.
IntraLase
STAAR Surgical Inc.
Iridex
Synergetics USA
ISTA Pharmaceuticals
TLC Vision
TASS Task Force Issues Final Report
No Single Cause Can Be Linked to Outbreak.
The Toxic Anterior Segment Syndrome (TASS)Task Force, sponsored by the American Society of Cataract and Refractive Surgery, has been meeting on an ongoing basis and compiling data in an effort to determine the cause of the increased incidence of TASS associated with cataract surgery that began in early 2006. The Task Force, which recently issued its final report, was not able to pinpoint a single cause for the TASS outbreak but did issue recommendations for operating room protocols that, if implemented, should reduce the incidence of TASS.
TASS is a postoperative inflammatory reaction caused by a noninfectious substance that enters the anterior segment, resulting in toxic damage to intraocular tissues. The process typically starts within 48 hours after cataract/anterior segment surgery, is limited to the anterior segment of the eye, is always Gram-stain and culture-negative and usually improves with steroid treatment.
The Task Force said a total of 113 surgery centers and hospitals reported cases of TASS from Jan. 17 to July 11, 2006, with many surgeons providing detailed data on these cases. The number of reported cases and completed surveys peaked in April 2006. Subsequently, the reported cases of TASS have decreased to what is considered a baseline level. While the Task Force will continue to evaluate potential etiologic factors involved in TASS, it appears as though the early 2006 outbreak has subsided significantly.
There were no conclusive epidemiologic data to suggest that any one product was responsible for the increase in TASS cases that were reported. Careful analysis of the information provided did not reveal a single cause or point source related to this TASS outbreak. The investigation failed to identify evidence supporting an association between a shared product and the cases of TASS reported. The Task Force said that failure to make that association does not mean that the association does not exist, nor does it mean that the increase in cases was due to a change in reporting. Either or both of these explanations might be correct, along with other hypotheses including those of a multifactorial nature.
Thorough analysis of the data provided has revealed multiple potential etiologic factors that could be involved in the outbreak of TASS. The Task Force said that the cleaning and sterilization of instruments for cataract surgery appears to be the most important factor involved in many of the cases of TASS reviewed. In addition, all of the products involved in preoperative preparation of the patient through postoperative care were reviewed and several potential factors identified as possible causes of TASS. The results of this investigation were shared with representatives of the FDA and the U.S. Center for Disease Control.
The TASS Task Force arrived at the following conclusions:
► While no single factor was found to be responsible for this outbreak of TASS, multiple potential etiologic factors were evaluated by the Task Force. Among potential contributing factors are reuse of single-use instruments, inadequate cleaning/rinsing of instruments, inadequate cleaning of ultrasound baths, failure to use sterile, deionized distilled water for flushing and use of medications with preservatives, bisulfites or metabisulfites. Task Force recommendations are aimed at preventing these occurrences.
► In addition, evaluation of outcomes in the majority of cases involved in this outbreak has shown that the degree of inflammation in these patients was rated as moderate in most cases. The majority of patients have recovered good visual acuity without significant ocular sequelae from the TASS. A small group of patients who had more severe inflammation have been reported to have ongoing ocular problems from the toxic insult.
► The Task Force will continue to evaluate data that is submitted and will also continue meeting by conference call on a regular basis. During visits by nurse consultants to more than 17 sites experiencing TASS, issues with cleaning ophthalmic surgical instruments were a common observation.
► symposium was held at Emory University that brought together many members of the Task Force as well as nurses and other personnel involved in the cleaning and sterilization of ophthalmic surgical instruments. The purpose of the meeting was to discuss the establishment of guidelines for the cleaning, sterilization and processing of ophthalmic instruments with the goal of preventing future TASS outbreaks. It is the intent of the Task Force to help in the establishment of these guidelines.
GIVING
BACK:The Armenian Eyecare Project
One
Doctor's Initiative Led to Major Successes.
By
Jerry Helzner, Senior Editor
When the Soviet Union broke apart in 1991, Armenia was able to establish its independence. But autonomy did not come without a price. Neighboring Azerbaijan attempted to annex Karabakh, a region in which Armenians make up the majority of the population. This dispute led to war.
"Casualties from the war began to overwhelm the Armenian healthcare facilities in 1992," recalls Roger Ohanesian, M.D., who was one of the first doctors to respond to the Armenian government's call for outside help. "I had never been to Armenia. I didn't speak the language. I wasn't even exactly sure where it was."
Dr. Ohanesian quickly made travel arrangements while also gathering up $10,000 worth of basic ophthalmic supplies donated by Alcon.
"I went to the country's major eye hospital in the capital city of Yerevan," says Dr. Ohanesian. "The conditions were really dismal. They were sorely lacking equipment and what they had was old. Their Bulgarian sutures often broke during a procedure. Being part of a closed society, the Armenian doctors had only learned Russian techniques, which were archaic and often just wrong."
But Dr. Ohanesian was greatly touched by two of his patients. He took one young man with severe shrapnel injuries to his eyes back to the United States and successfully restored his sight to 20/25. He operated twice on a young girl who was almost blind from a form of childhood uveitis. When the operations failed, he enlisted the aid of Richard Hill, M.D., then of the University of California, Irvine, who flew to Armenia to perform a successful procedure.
|
|
|
Dr. Ohanesian examines an elderly Armenian woman. |
"Dr. Hill was the first of many volunteer ophthalmologists who have helped in Armenia," says Dr. Ohanesian. "We have had a total of about 50 in the 14 years that we have been doing this work. Many of them, like Dr. Hill, have made numerous trips and many are not of Armenian descent."
As Dr. Ohanesian continued to make trips to Armenia, he began to formalize the effort into the Armenian Eyecare Project (AECP), with a focus on bringing leading academic ophthalmologists to teach Armenian doctors how to perform procedures using the latest western techniques and modern equipment.
"We provided promising Armenian ophthalmologists with 1-year fellowships in the United States in all subspecialties," he notes. "And when they went back to Armenia, they were provided with the best equipment and supplies, approximately $20 million worth at last count."
This approach has been so successful that Armenia has been transformed into a center of eyecare excellence, with other countries sending ophthalmologists to train in Yerevan.
"In 2003, we focused on bringing eye care to the more remote areas of Armenia," says Dr. Ohanesian. "For this, we converted an 18-wheel tractor trailer into a mobile eye clinic. Armenian ophthalmologists can now cover all areas of the country, performing screenings, advocating more healthy lifestyles and treating the people who need it. We have greatly reduced the incidence of blindness in the country by removing cataracts, and by treating glaucoma, diabetic retinopathy and other diseases that can lead to blindness."
The AECP has captured the imagination of Armenian-Americans, who have donated generously to the effort.
"We enlist donors in more than giving money," says Dr. Ohanesian. "If they have ties to a particular area of the country, they can take part in our Adopt-A-Village or Adopt-A- School programs. In return, they receive photos and tapes from the people they are helping. There is a real connection."
AECP has become a large, sophisticated nonprofit organization with a professionally edited quarterly newsletter and mailers to donors that recount treatment successes.
But AECP is not resting on its record of accomplishments.
"Our latest initiative is to teach 2,500 general practitioners
in Armenia the basics of ophthalmology so they will be better able to detect eye
disease," says Dr. Ohanesian. "At the same time, we are teaching the country's rural
ophthalmologists how to treat and
follow-up."
IN THE NEWS
■ AMO sees profit shortfall. Advanced Medical Optics, Inc. (AMO) said slower adoption of refractive implants by surgeons outside the United States, a sluggish domestic laser vision correction market and reimbursement issues in Japan and parts of Europe have caused the company to revise guidance for 2006 earnings per share downward to a range of $1.90 to $1.95, compared to prior guidance of $2.05 to $2.21. AMO now expects 2006 revenue to be in the range of $1,010 million to $1,020 million, compared to prior guidance of $1,020 million to $1,040 million.
For 2007, the company expects revenue to be approximately $1,100 million, compared to prior guidance in the range of $1,100 million and $1,120 million, and adjusted earnings to be approximately $2.60 per share.
"Recent market conditions have made it difficult for us to move as quickly as we had planned to improve our sales mix and deliver the full level of 2006 margin expansion previously projected," said Jim Mazzo, AMO chairman, president and chief executive officer. "While we are disappointed by this near-term change in our outlook, we are experiencing significant progress toward achievement of our key metrics and remain confident in our ability to deliver our 2007 guidance."
■ Violet-filtering IOL. Bausch & Lomb has launched the SofPort AO Violet-Filtering Aspheric Lens with advanced light-blocking technology. This technology prevents harmful, high-energy light from reaching the retina without compromising the patients' low-light color vision. The company claims that the new violet-blocking IOL provides better functional vision than lenses designed to block blue light.
■ BAK-free Travatan. Alcon announced that the FDA has approved Travatan Z (travoprost ophthalmic solution 0.004%) for the reduction of elevated IOP in patients with open-angle glaucoma or ocular hypertension, who are intolerant of or insufficiently responsive to other IOP-lowering medications. Travatan Z is a new formulation that eliminates benzalkonium chloride (BAK) from Alcon's existing Travatan solution and replaces BAK with SofZia, a robust ionic-buffered preservative system that Alcon says is gentle to the ocular surface. Alcon says it developed this BAK-free version of Travatan because long-term use of topical solutions containing BAK may compromise the ocular surface and exacerbate conditions such as dry eye.
"Because almost 40% of glaucoma patients suffer from ocular surface disease, Travatan Z is an advance in therapy which we believe will now enable doctors to address an unmet need of many glaucoma patients," said Kevin Buehler, Alcon's senior vice president, United States, and chief marketing officer.
FDA approval of Travatan Z solution was based on a double-masked, multi-center study which has been accepted for publication by the Journal of Glaucoma. The 690 adult patients with open-angle glaucoma or ocular hypertension were randomized to receive Travatan or Travatan Z. Travatan Z reduced IOP up to 8.5 mm Hg on average demonstrating statistically equivalent IOP-lowering efficacy to the original Travatan. Similar adverse events were noted in both groups.
■ Lucentis/Avastin trial. A large-scale head-to-head study comparing the merits of Lucentis and Avastin as intravitreal treatments for wet AMD will become a reality. The National Institutes of Health (NIH) will provide the financial support for the study of the two Genentech therapies, which have both proved effective in improving the vision of many wet AMD patients. There have been many calls for such a study due to the fact that FDA-approved Lucentis costs approximately $1,950 an injection and the cancer treatment Avastin can be given off-label for less than $100 an injection.
The trial is expected to cost about $16 million and involve 1,200 patients.
In related news, Genentech said that sales of Lucentis were $153 million in the quarter ended Sept. 30. Wall Street analysts had been estimating sales at between $35 and $45 million.
■ P4P update. The ophthalmology community has taken a major step toward allowing ophthalmologists to participate in Medicare pay-for-performance differentials, provided Congress passes legislation this year. Pending versions of Congressional Sustainable Growth Formula (SGR) fixes would establish differentials for reporting on quality measures, including a version that would take effect as early as 2007.
In October, eight ophthalmologic measures, including those for glaucoma, AMD, diabetic retinopathy and cataracts, were approved by the American Medical Associations Physician Consortium for Performance Improvement (PCPI). This action increased the likelihood of eye care inclusion in any quality reporting bonus pool that might be created.
■ Lid-scrub study. According to Cynacon/OCuSOFT, a recent time-kill study conducted by Care BioPharma found that OCuSOFT Lid Scrub Plus Extra Strength showed a 5.5 log reduction of Staphylococcus epidermidis, while a competing scrub's rate measured a 3.5 reduction. The measurements were taken after both products were subjected to the pathogen for 60 seconds.
A separate study on irritation by Tox Monitor Laboratories placed Lid Scrub Plus in the "Practically Non-Irritating" category, an entire rating category milder than the competitor. Cynacon/OCuSOFT expects to formally introduce Lid Scrub Plus during the American Academy of Ophthalmology meeting this month.