Cleaner
LASIK: Is it Possible? (Part I)
Risks
for contamination exist. The challenge is to eliminate them.
BY L.C. LAHAYE, M.D., H.H. RIEKE,
PH.D. AND F.F. FARSHAD, PH.D.
GRAPHICS COURTESY OF L.C.
LAHAYE, M.D.
One of the ultimate goals in performing any surgical procedure is to minimize less-than- desirable outcomes arising from both infectious and noninfectious contaminants entering the surgical field. This is especially true of all corneal procedures, such as LASIK, where the normal mechanisms for fighting contaminations are diminished.
Most patients requesting refractive surgery are relatively young and healthy, thereby minimizing the possibility of having systemic diseases which would impede surgical success. Such complications fall outside the discussion of standardized procedures that should result in a cleaner LASIK procedure. This article will point out areas of the LASIK procedure that currently present risks for contamination and also provide recommendations for reducing those risks.
|
|
|
|
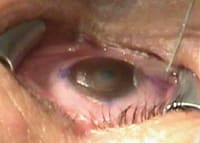
Figure 1. Saccadic movements can lead to inadvertent
contact of the target stroma with the lid margins and surgical drapes, resulting
in contamination and unwanted hydration and its negative impact on laser effectiveness. |
|
LASIK Stages 1 and 2
Stage 1 of LASIK involves the automated mechanical or laser-performed keratotomy that presents the surgeon in theory with a closed, noncontaminated surgical incision.
Stage 2 of the operation is much more dynamic and involves a multiplicity of procedures that require consistency, standardization and strict adherence to basic surgical principles and techniques to avoid less than desired outcomes. The excimer laser dose delivery is only one aspect of the more technically demanding Stage 2, which begins the moment the corneal flap is reflected and is completed with the corneal flap returned and sealed in its original position. During this stage, direct contact with lid margins, lashes, surgical drapes and invasive solids and fluids can arise owing to the involuntary introduction and inclusion of infectious organisms, epithelial cells, debris, oils and tear duct secretions into the stroma after the corneal flap is reflected back off the corneal surface. Manipulations of both epithelial and stromal surfaces of flap and bed, introduction and extraneous use of instruments to the interface, flap folding, coupled with inefficient plume evacuation, nonuniform and varying hydration of the target stroma, stromal bleeding, prolonged flap adherence time, involuntary saccadic and cyclorotational ocular movements throughout the second stage of the LASIK procedure may each contribute to less than desirable outcomes (Figure 1).
Moreover, lack of, or inefficient, removal of plume can lead to deposition of smoke particles on the laser's exposed optics, increasing the need for cleaning or replacement. It has been widely observed by surgeons that sometimes the generated plume carries large particles, which could drop out onto the surgical field creating additional contamination in the region of the incision and/or these particles adhere after splattering onto the laser's last optic resulting in irregular etching. Both events cause grief for the surgeon and patient and may require complex surgical intervention in an attempt to correct poor outcomes. Additionally, splatter, smoke and large particles are possible health and safety issues for the surgeon, medical staff and patient.
Identifying Contamination Risks
How can we improve LASIK surgical method to reduce contamination complications? This appears to be a difficult challenge owing to the variability among surgeons' practice and skills. Before specifically addressing this question, we should review the nine important operational functions that a surgeon has to handle in the second stage of LASIK.
Containment of the surgical field. The surgical field boundaries of the standard LASIK procedure include the patient's lid margins, lashes, cul de sac tissues and surgical drape if used. These regions can be sources of contamination. It is known that the exposed corneal stroma has the potential to absorb and hold invasive fluids like a sponge. Buratto et al,1 and Pushker et al,2 pointed out that a reduction in the exposure of the cornea bed and flap tissues can reduce postoperative complications, including infectious keratitis and diffuse lamellar keratitis (DLK).
A cleaner LASIK procedure would require the downsizing as well as the containment of the surgical field to reduce exposure of the cornea bed and flap tissues to contamination and thereby reduce the incidences of possible occurring infectious keratitis, DLK and interface debris.
Fixation and control of eye movements. Fixation of the patient's eye is problematic in conventional LASIK surgery. With respect to this function, there are three main areas of concern.
The first involves the current standard procedure and the use of various surgical tools which unfortunately do not downsize the surgical field nor provide for containment of the delicate and highly absorbent flap and stromal bed tissues. In addition to alignment issues, saccadic movements can lead to inadvertent contact of the target stroma with the lid margins and surgical drapes, resulting in contamination and unwanted hydration that has a negative impact on laser effectiveness.
During ablation, excimer laser beam tracking has limitations in that there will always be a critical delay between measurement alignment and delivery. Even with high-tracking sampling rates at 4,000 times per second, time is needed to adjust the laser mechanics and optics to ensure proper energy delivery to the predetermined cornea target site.
A second limitation of most trackers is the inability to actively track cyclorotation movements of the eye, which can contribute to inaccurate placement of the laser. This misalignment of the axis during laser delivery can result in poor visual outcomes with increased higher-order aberrations and loss of BCVA. The third limitation of trackers is the "false sense of security" created by the technology, causing some surgeons to take a "back seat" approach to the laser delivery step, allowing tracker "drift" to go unnoticed. Any approach to improving LASIK outcomes will have to address these areas of concern.
|
|
|
|
Figure
2. During ablation, the highly absorbent flap is unprotected "marinating" in fluids
and secretions that contain debris, oils, and other contaminants from direct contact
with the conjunctiva, lid margins, surgical drape and lashes. |
Corneal flap management. After performing a keratotomy, in theory the surgeon is presented with a closed, noncontaminated corneal incision. The flap must be reflected to expose the underlying stromal bed that is the target to be corrected by laser removal of tissue, which reshapes the curvature of the cornea. Typically, the flap is reflected open or sometimes folded in half ("taco" technique) and then flayed either directly on the eye (nasal or temporal hinge), lid margin region (superior flap hinge) or on or under a surgical sponge or metal tool where it remains through out the laser delivery.
During this period, the highly absorbent flap may be subjected to mechanical stress. In addition, the flap may be exposed "marinating" in fluids and secretions that contain debris, oils, and other contaminants from direct contact with the conjunctiva, lid margins and lashes. (Figure 2) None of the aforementioned methods provides for containment of the flap. Cleaner LASIK would eliminate flap-reflection uncertainties.
Removal of flap-bed surface fluid/moisture. Surgeons must rely on a variety of techniques and devices if they are to be able to modify and adjust for dynamic changes relative to hydration variability on the target stromal tissue surface during laser pulse delivery.
Stromal fluid can mask the effectiveness of the laser energy's ability to remove tissue, causing variations in ablation that can result in "islands" and under corrections.3,4 Standard procedures are to either to wipe the surface using a microsponge or metal spatula, and/or employ airflow to evaporate the excessive moisture from the stromal bed prior to and during ablation. Unfortunately, the microsponge leaves the stromal surface visibly grainy and rough and sometimes leaves particles. A metal instrument and dry sponge can also create abrasions at the margins of the flap bed, which have been implicated in epithelial ingrowth.
Some conventional LASIK procedures use airflow through tubing — that may or may not be filtered or sterile — to the corneal surface during or prior to ablation to minimize uneven and changing hydration conditions that could result in central islands or undercorrections. A cleaner LASIK procedure would reduce the possibility of surgical enhancements or revisions due to hydration variability during and prior to ablation while not contributing to additional complications.
Plume evacuation. Plume generated during LASIK surgery can present several potential troublesome operational outcomes and patient/surgeon health problems. Plume is created when the excimer laser pulse strikes cornea water vapor and live and dead cellular debris located in the surgical field. Ejection of the biocomponents is due to the resulting photomechanical effects of UV-energy transfer to tissue at the corneal surface. The ablation process breaks the nitrogen peptide bonds in cellular proteins generating plume "smoke," an aerosol that can result in a beam-blocking effect as the plume hangs over the ablating stromal bed blocking subsequent laser pulses. The plume composition includes water vapor, cellular and carbonized tissue, blood and viruses in conjunction with benzene, hydrogen cyanide, toluene gases, formaldehyde and polycyclic aromatic hydrocarbons.
The "burning flesh" odor resulting from the excimer laser beam is strong and stenchful to the physician, nurses and patients in the operating room. In addition, the aerosol plume particles attach to hair, clothing, surfaces of surgical equipment and exposed skin, and can heighten patient anxiety.
The mechanisms involved in plume interference begin with the molecular dynamics of tissue ablation and its consequence in creating airborne biological particle ejection. The plume consists of monomers, molecular clusters, large molecules and fractured tissue fragments by the mechanisms of desorption, melting, hydrodynamic sputtering, vaporization, tissue explosion due to overheating and photomechanical exfoliation and spallation.5
These particles form a cloud between the laser and stromal bed causing a masking interference in the beam's ability to properly etch the stromal optical zone. Yingling et al.6 research based on computer model simulated laser ablation of biocomponents systems revealed that molecular ejection mechanisms favored volatile solutes to be ejected mainly as monomers, whereas the nonvolatile components tend to form clusters during ejection. Systematic large-scale molecular dynamic computer modeling studies have investigated the influence of the role of laser pulse duration, fluence and wavelength, laser spot size, number of successive laser pulses, laser beam incidence angle, temperature effect on the molecular substrate and molecular volatility to comprehend the laser ablation phenomenon.
The complexities of plume formation and its rapid dynamic movements both vertically and laterally impose problems that can present undesirable outcomes.
There is considerable variability in LASIK surgery in the way that excimer-generated plume is managed. Standard LASIK plume management is typically based on the evacuation of the plume with large-volume laser integrated plume evacuation systems. The large size of laser-integrated plume evacuators limits how close they can be positioned to the source of plume during ocular surgery. Research has demonstrated that the efficiency of plume evacuation degrades rapidly the farther the evacuation port is from the target tissue. Some researchers believe the plume evacuators integrated into the commercial existing excimer lasers systems are extremely inefficient.7 Physicians and patients question the effectiveness of the evacuators on some lasers.8
|
|
|
|
Figure
3. Cleaner LASIK outcomes will require an efficient plume evacuation system that
not only reduces the incidence of beam masking and plume splatter attaching to the
laser's last optic, but also allows for improved control of dehydration during evacuation. |
Cleaner LASIK outcomes will require an efficient plume evacuation system that not only reduces the incidence of beam masking and plume splatter attaching onto the laser's lens, but also allows for improved control of dehydration during evacuation (Figure 3).
Irrigation. Surgeons must perform irrigation of the corneal surface using sterile fluids multiple times during LASIK surgery. Irrigation is used to: wet and lubricate the cornea before keratotomy, hydrate the tissues, rinse laser and keratome generated micro-debris from the surgical zone before flap repositioning, and facilitate refloating the flap back into its original position.
Currently, the irrigation procedure is accomplished using various individual devices requiring extensive use of manipulations. Problems arise when excessive irrigation fluids backwash and collect forming a "lake" in the nasal or temporal canthal triangle and mix with the conjunctiva, lids and fornix areas, requiring the introduction and application of sponges. The pooling of fluids can be a contributing source of infectious and noninfectious contamination even after washing the surface with betadine and antibiotic solutions. Also, any backwashing of the pooled irrigation fluids into the stromal bed and flap increases the risk of infectious/inflammatory complications. The exposed corneal stroma of both the flap and the bed absorb fluid readily, like a sponge. This condition is analogous to trying to remove soap from a sponge, even with repeated rinsing and squeezing.
|
|
|
|
Figure
4. To achieve a cleaner LASIK irrigation procedure will require less instrumentation
and accompanying manipulations, coupled with a method that allows for generous (unlimited),
nonturbulent sterile irrigation without concern for backwash onto the surgical site. |
|
Some surgeons attempt to avert backwash by minimizing irrigation, but the downside here is not providing sufficient fluids to adequately rehydrate or thoroughly rinse and wash the flap bed, thus contributing to flap striae or interface complications. In a cleaner LASIK, irrigation procedure will require less instrumentation and accompanying manipulations, coupled with nonturbulent laminar sterile irrigation without backwash onto the surgical site (Figure 4).
Aspiration. During LASIK surgery, effective removal of irrigation and tissue fluids can reduce backwash of micro-debris, contaminants and foreign material onto the exposed stromal bed as well as the corneal flap.
How is aspiration of these invasive contaminants handled at present? In conventional LASIK practice, the fluid is allowed to reach a level where it runs off the surgical field if it has not been effectively removed by an aspirating lid speculum or absorbed by sponges. Karp et al,9 stated that it is necessary to remove pooling fluids by absorption or mechanical means to reduce the levels of fluid exposure in the surgical field and to minimize infectious keratitis after surgery.
The use of a surgical sponge helps to reduce fluid pooling. Microsurgical sponges, however, are limited by their absorption capacity, and retain and concentrate fluids in or near the surgical field. In addition, sponges tend to rough up the exposed stromal tissue. With respect to aspirating lid speculums, they have reduced efficiency in deepset eyes and cannot prevent backwash of fluids onto the exposed stromal tissues. Cleaner LASIK will mandate that these issues be resolved, perhaps by a better irrigation and aspiration design that minimizes flap manipulations and use of extraneous instrumentation while simultaneously guarding against backwash.
Flap repositioning and realignment. Ophthalmic surgeons Belda et al,10 Pushker et al,2, Rojas and Manche,11 and Stewart12 hold the opinion that reducing manipulation and exposure of the cornea bed and flap tissues to cul-de-sac fluids and lid margins could reduce some postoperative complications. It is standard in LASIK procedure to reflect the flap over onto the flap bed by a series or combination of multiple flap manipulations using surgical forceps, spatulas and/or cannula. After the flap has been reflected onto the bed using additional manipulations, it is common practice for an irrigation cannula connected to a manual squeeze bottle or syringe to be inserted between the flap and its bed. This added manipulation delivers uncontained irrigation to float the flap allowing the surgeon to smooth out and align the flap back into its original position.
Irrigation fluids add to the pooling and backwashing of surgical fluids and increase the required time and manipulation needed to dry and fixate the flap. The fluids can pool and mix with the lid margins and lashes and backwash cellular debris into the flap-bed interface, becoming permanently trapped. Any material left in the interface has the potential to cause DLK, infectious keratitis and can also contribute to epithelial undergrowth.13-15
To improve the flap repositioning and realignment procedure, we must find a way to reduce the number of flap manipulations. Additionally, a means has to be designed to keep either the flap out of the fluid pools that harbor debris and contaminates, or eliminate the fluid, or both so that a cleaner surgical field will result in a cleaner procedure.
Flap adherence. It is critical the corneal flap be uniformly adhered to the corneal surface following assured repositioning and alignment to reduce flap complications such as macro/micro striae and epithelial ingrowth.
After flap replacement, the surgeon normally observes the flap from 3 to 5 minutes, allowing the flap to adhere. Some surgeons may use a surgical sponge to "dry the gutter" or squeegee the flap in an attempt to shorten the adherence time. This action may shorten the time, but a sponge is rough and excessive use can create or extend abrasions along the flap surface and margins that may be contributory to flap-related complications. In as much as the surgical field in conventional LASIK is uncontained, the sponge may also contact and absorb surgical fluids and cellular debris. It is possible that the flotsam can be inadvertently painted over the flap tissues or a micro-abrasion from the keratotomy can be made worse with repeated sponge use when squeezing the flap down and out.
Perez16 demonstrated that air-drying across the repositioned flap increases stromal-stromal adhesion. Airflow across the repositioned flap accelerates flap adhesion and shortens surgery time, replacing the 3-to-5-minute adhesion wait time. Researchers have demonstrated that this method can effectively and safely replace the "old standard of waiting 3 to 5 minutes for flap adhesion" and that it also "allows for control of flap drying in a uniform manner."17,18.
A cleaner LASIK procedure will have to improve the method of providing micro- filtered sterile, laminar airflow to the realigned flap so that the stromal-to- stromal adherence can be safely accelerated and the flap dried in a uniform manner. In addition, cleaner LASIK will minimize or eliminate the uncontained use of surgical sponges.
The Next Advance
Over the past 10 years, LASIK surgery has improved owing to advancements in excimer laser and keratome technology, yet the incidence of nonlaser- and nonkeratome-related complications and less-than-desired outcomes requiring additional medical and surgical intervention remains statistically significant. The only other avenue of substantial improvement has to come through the design improvement process of surgical methods and devices that are aimed at reducing the majority of persistent complications associated with laser refractive procedures.
In Part 2, the authors will discuss cleaner LASIK by design.
Leon C. LaHaye, II, M.D., is the medical director of LaHaye Total Eye Care in Lafayette, La. His e-mail is ifxiis@lahayesight.com. Herman H. Rieke, Ph.D., is professor of petroleum engineering at the University of Louisiana at Lafayette. Fred F. Farshad, Ph.D., is a Chevron-endowed research professor in the department of chemical engineering at the University of Louisiana at Lafayette.
References
1. Buratto L, Brint SF. Custom LASIK: Surgical Techniques and Complications. Slack Inc., Thorofare, NJ. 2003;816.
2. Pushker N, Dada T, Sony P, Ray M, Agarwal T, Vajpayee RB. Microbial keratitis after laser in situ keratomileusis. J Refract Surg. 2002;18(3):280-286.
3. Dougherty PJ, Wellish KL, Maloney RK. Excimer laser ablation rate and corneal hydration. Am. J Ophthalmol. 1994;118(8):169-176.
4. Oshika T, Klyce SD, Smolek MK, McDonald MB. Corneal hydration and central islands after excimer laser photorefractive keratectomy. J Cataract Refract Surg. 1998;14(12):1575-1580.
5. Zhigilei LV. Dynamics of the plume formation and parameters of the ejected clusters in short-pulse laser ablation. Appl Phys A. 2003;76:339-350.
6. Yingling YG, Zhigilei LV, Garrison BJ. Laser ablation of biocomponents systems: a probe of molecular ejection mechanisms. Appl Phys Letters.. 2002;78(11):1631-1633
7. Dell S. New system permits safe, more effective plume evacuation. Ophthalmology Times. 2003;28(8):48.
8. Piechocki M. Lack of data complicates concerns over LASIK surgical smoke. Ocular Surgery News. 2002;20(24):64-67.
9. Karp C, Tuli SS, Yoo, SH, Vroman DT, Alfonso EG, Huang AH, Pfugfelder SC, Culbertson WW. Infectious keratitis after LASIK. Ophthalmology 2003;110(3):503-510.
10. Belda J, Artola A, Also J. Diffuse lamellar keratitis 6 months after uneventful laser in situ keratomileusis. J Refract Surg. 2003;19(1):70-71.
11. Rojas M, Manche E. Early diagnosis, treatment essential when facing DLK. OphthalmologyTimes. 2001;26(17):5-8.
12. Stewart P. Sands of the Sahara. In: Buratto, L. and Brint, S., eds, LASIK: Surgical Techniques and Complications, 2nd Edition, Slack Inc. Thorofare, NJ, 2003:597-598.
13. Haddril M. Prolonged sterilization may be needed for killing endotoxins. EyeWorld. 2002;7(5):31-32.
14. Lipner M. The opportunists: Infectious organisms cozying up to LASIK. EyeWorld. 2002;7(3):22-27.
15. Naoumidi I, Papadaki T, Zacharopoulos I, Siganos C, Pallikaris I. Epithelial ingrowth after laser in situ keratomileusis. Arch Ophthalmol. 2003;121(7):950-955.
16. Perez E. Factors affecting corneal strip stroma-to-stroma adhesion. J Refract Surg. 1998;14:460-462.
17. Pascucci S. Flap creation and management strategies. Ophthalmology Management. 2002;6(9):S10-S11.
18. Schumer DJ and Schumer J. Maximizing visual recovery in LASIK surgery. Cataract and Refractive Surgery Today. 2002; 2(4): 53-54.