Preventing Post-Cataract Extraction CME
Early identification of patients at risk and
prophylactic treatment may avert vision loss.
BY JEFFREY S. HEIER, M.D.
Cystoid macular edema (CME), first recognized more than 40 years ago, is the leading cause of compromised visual acuity following cataract extraction. Despite physician vigilance and the availability of adequate prophylaxis, CME is still routinely encountered. In the past 2 years alone, 25 articles have been written about this pervasive condition.
In this article, I discuss how to identify at-risk patients and the use of topical nonsteroidal antiinflammatory drugs (NSAIDs) for preventing and treating CME.
|
|
|
|
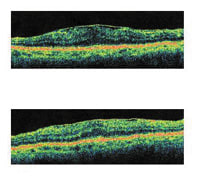
The FA on the top shows a patient post-laser treatment for macular edema secondary to a BRVO with resolution of edema. The OCT scan on the
bottom shows normal anatomy without macular edema. |
|
CME Can Lead to Significant Vision Loss
CME is characterized by a yellow cystic appearance to the fovea, which may or may not be appreciated on slit lamp examination using a fundus lens. Fluorescein angiography, considered the gold standard for diagnosis, will reveal early perifoveal hyperfluorescence with late leakage in a petaloid pattern. The optic nerve is usually hyperfluorescent as well.
Clinical CME has the same characteristics plus some degree of vision loss, typically 20/40 or worse. Angiographic CME is often described as having the same characteristics but without vision loss, at least not to the 20/40 level. In the past, angiographic CME was not considered significant in terms of vision loss. But with advances in surgical techniques and instrumentation, both patients and surgeons now have a higher level of expectation as to outcome. The end result is a change in what is considered clinically significant, with any decrease in vision, even at levels of 20/30 or better, now considered significant.
Clinical pseudophakic CME, which is characterized by decreased visual acuity, often with loss of contrast sensitivity, and metamorphopsia, ranges in reported incidence in the literature from 0.2% to 12.1% depending on the study design and definition of CME. Assuming 2 million surgeries annually without any type of prophylactic intervention, this amounts to 4,000 to 240,000 new cases annually. So this form of CME is of clinical consequence.
Natural history studies of pseudophakic CME have shown that a majority of cases resolve spontaneously, although this resolution often took months, and visual acuity loss was the only endpoint studied. If untreated and allowed to persist for months, retinal changes and irreversible vision loss can occur and may manifest as metamorphopsia, contrast sensitivity loss, or other less-measured parameters of vision.
|
|
|
|
The top OCT scan shows a post-cataract patient with CME and an epiretinal membrane. The bottom OCT scan shows the same patient with decreased macular edema post-treatment with prednisolone and ketorolac
q.i.d. for 10 weeks, then tapered. The epiretinal membrane is still present and causing retinal thickening. |
|
Assessing Risk
The risk of CME doesn't arise solely from the cataract procedure itself. Each patient introduces an idiosyncratic set of circumstances that will affect his or her susceptibility to CME. The challenge for the surgeon is to identify those circumstances and then assess the risk. Taking a thorough medical history and doing a careful ophthalmologic examination should reveal a patient's risk factors. Preoperative prophylaxis can then be started for that patient. Many anterior segment surgeons have adopted a preoperative regimen for all patients undergoing cataract surgery to decrease the likelihood of CME.
Risk Factors for CME
Several factors associated with cataract surgery may play a role in post-surgery CME, presumably the result of inflammatory reactions. These include complicated extraction, capsular rupture, use of an iris-fixated lens, ruptured hyaloid face, iris incarceration, vitreous loss, presence of epiretinal membranes, and vitreomacular traction syndrome. Even the trauma of uneventful surgery may be a factor.
Certain pre-existing conditions can also predispose patients to CME:
Ocular inflammation. Pre-existing ocular inflammation in any of its many forms -- even in the fellow eye -- is the single most prevalent risk factor. Inflammation may take the form of idiopathic uveitis, intermediate uveitis, birdshot retinochoroidopathy, sarcoidosis, toxoplasmosis, posterior scleritis, Harada's syndrome, pars planitis, and Behçet's syndrome. Common to all of these is inflammatory-mediated breakdown in the blood-retina barrier (BRB). Ocular inflammation is a common cause regardless of whether or not cataract surgery has been performed. Patients who have a history of uveitis-associated CME appear to be at greater risk of developing CME post extraction, even with uncomplicated surgery. Treatment for these patients can be extremely frustrating as they are often refractory to common interventions; therefore, careful control of any pre-existing inflammation and prophylaxis are critical.
Diabetes. It's estimated that in the United States, 4.1 million adults 40 years of age or older have diabetic retinopathy, the most important risk factor for CME outside of cataract surgery. Although most anterior segment surgeons are aware that cataract surgery in patients with diabetic retinopathy poses a risk, many are unaware that even patients without any preoperative evidence of diabetic retinopathy are also at significant risk. For them, the incidence of postsurgical CME is increased, with development of the condition seen in 16% to 56% of such patients. CME spontaneously resolves in approximately 50% to 60% of patients with diabetic retinopathy when it occurs post extraction but virtually never if it's present at the time of surgery. When CME occurs in this setting, medical treatment is often unsuccessful, so prevention should be the primary goal for diabetic patients.
Ocular vascular disease. Venous occlusive disease may lower the threshold for BRB breakdown, and the risk appears to be greatest in patients with a history of prior macular edema. Both central retinal vein occlusion and branch retinal vascular occlusion are risk factors, as is retinal arterial disease, both branch and central retinal artery occlusions. Unfortunately, small or resolved branch retinal vein or artery occlusions may go unnoticed and only manifest as unexplained visual loss. A careful preoperative evaluation with close attention to unexplained visual deficit (out of proportion to the degree of cataract) is critical to detect deficits that won't improve with cataract extraction and to recognize any CME risk.
Less-common retinal vascular conditions also place cataract patients at greater risk of CME. These include retinal telangiectasis (Coats' disease, radiation retinopathy, and idiopathic retinal telangiectasia) and several forms of retinal vasculitis (Eales' disease, Behçet's syndrome, sarcoidosis, necrotizing angiitis, multiple sclerosis).
Cardiovascular disease. Ischemic heart disease may predispose a patient to CME. A retrospective review of 252 patients who underwent cataract extraction showed that CME occurred in 4 patients who had ischemic heart disease. Although not conclusive, this is cause for thought and is particularly noteworthy because ischemic heart disease is most prevalent in the same geriatric population in which most cataract procedures are performed. Until further studies are performed, these patients should be considered potentially high-risk and have routine prophylaxis.
Inherited disease. Patients with retinitis pigmentosa have an increased incidence of CME. Although less is known of the mechanism of this cause, prophylaxis would be beneficial.
Ocular or systemic medications. The use of certain medications such as epinephrine, dipivefrin, vancomycin (infusion during cataract surgery), or prostaglandin analogues have been associated with CME. Epinephrine is implicated presumably due to its ability to reduce blood flow to the retina and choroid. Prostaglandin analogues may disrupt the blood-aqueous barrier, increasing the likelihood of CME after cataract surgery. However, recent reports raise questions as to whether this is due to the increased prostaglandin load introduced by the active agent or a reaction to a preservative used in the drug formulations.
Other factors. Previous ocular surgery, with retinal procedures more frequently implicated than those for the anterior segment, is a factor as is radiation treatment within the previous 3 years involving the head and neck.
|
|
A rational plan for NSAID prophylaxis of pseudophakic clinical CME. Preoperative dosing for a longer period would be necessary in patients with confirmed risk factors. |
|
Preoperative: Routine Patients Preoperative: High-Risk Patients Postoperative: All Patients
(high-risk patients) |
NSAIDs as Prophylaxis for CME
Historically, corticosteroids have been the drugs of choice for preventing or treating postoperative ocular inflammation. Although effective, these drugs may interfere with wound healing, aggravate infection, or elevate IOP.
Topical ophthalmic preparations of NSAIDs have now been welcomed by ocular surgeons as effective alternatives for controlling inflammation and are widely used (alone or in combination with steroids) to prevent and treat CME after cataract surgery. NSAIDs decrease prostaglandin synthesis by inhibiting cyclooxygenase, thus preventing the transformation of arachidonic acid into prostaglandins.
Unlike corticosteroids, topical NSAIDs have multiple functions. They were first used in cataract surgery to prevent surgically induced miosis and reduce inflammation. They also effectively controlled the pain of refractive surgery and were found to decrease the postoperative inflammation that characterizes CME. It has also been shown that NSAIDs have the potential to prevent and treat CME. Most importantly, NSAIDs appear to be no less effective than some steroids for suppressing the inflammatory response and are beneficial in treating inflammation after intraocular surgery.
Topical NSAIDs have an advantage over corticosteroids for patients in whom steroids are contraindicated, including patients who are susceptible to corticosteroid-responsive IOP elevations, or have recurrent herpes simplex infection or delayed wound healing.
Although NSAIDs are widely recognized as providing most of the clinical benefits of steroids while avoiding the worst of their side effects, they do have limitations. Complications associated with the use of topical NSAIDs include ocular irritation and discomfort following application, conjunctival injection, mild punctate keratopathy, and mydriasis. Allergic and hypersensitivity reactions also have been reported. On balance, however, this safety profile is far more acceptable than that of the steroids.
The literature supports the efficacy of topical NSAIDs, such as flurbiprofen 0.03%, diclofenac 0.1%, and ketorolac 0.5%, used prophylactically after surgery to reduce many forms of ocular inflammation, including CME. One drop is administered q.i.d. for 3 to 6 weeks to prevent CME. In chronic cases, management continues until resolution. In some cases, indefinite NSAID treatment may be required to maintain CME regression.
Prevention is the Best Course
The ideal management of pseudophakic CME is to prevent it from ever occurring. Although this may be reasonable for all patients, it's particularly helpful for high-risk patients. One of the most rational and cost-efficient approaches to prophylaxis is to administer a topical NSAID preoperatively, maintain administration intraoperatively for prevention of miosis, and continue administration postoperatively to control inflammation and as additional prophylaxis of CME.
Dr. Heier is a vitreoretinal specialist at Ophthalmic Consultants of Boston, president of the Center for Eye Research & Education Foundation, and a clinical instructor in ophthalmology at Tufts University School of Medicine and Harvard Medical School.
|
Efficacy of Topical NSAIDs in Preventing and Treating
CME |
References 1. Solomon KD, Cheetham JK, DeGryse R, et al. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology. 2001;108(2):331-337. 2. Arshinoff SA, Opalinski YAV. The pharmacotherapy of cataract surgery. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 331-336. 3. Holzer MP, Solomon KD, Sandoval HP, et al. Comparison of ketorolac tromethamine 0.5% and loteprednol etabonate 0.5% for inflammation after phacoemulsification. J Cat Refract Surg. 2002;28(1):93-99. 4. Solomon KD, Vroman DT, Barker D, et al. Comparison of ketorolac tromethamine 0.5% and rimexolone 1% to control inflammation after cataract extraction. Prospective randomized double-masked study. J Cataract Refract Surg. 2001;27(8):1232-1237. 5. Flach AJ, Stegman RC, Graham J, et al. Prophylaxis of aphakic cystoid macular edema without corticosteroids. A paired-comparison, placebo-controlled double-masked study. Ophthalmology. 1990;97(10):1253-1258. 6. Flach AJ, Jampol LM, Weinberg D, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol. 1991;112(5):514-519. 7. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107(11):2034-2038;discussion 2039. 8. Rho DS. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg. 2003;29(12):2378-2384. 9. Yeh PC, Ramanathan S. Latanoprost and clinically significant cystoid macular edema after uneventful phacoemulsification with intraocular lens implantation. J Cataract Refract Surg. 2002;28(10):1814-1818.
|
|
Efficacy of Topical NSAIDs in Preventing and Treating CME |
References 1. Quentin CD et al. Prophylactic treatment of cystoid macular oedema with diclofenac eyedrops in intracapsular cataract extraction with Choyce Mark IX anterior chamber lens. (In German) Fortschr Ophthalmol 1998;86:546-549. 2. Rossetti L et al. Effectiveness of diclofenac eyedrops in reducing postoperative inflammation angiographic CME after cataract surgery. J Cataract Refractive Surg. 1996;22S:794-799. 3. Gallenga PE et al. Efficacy of diclofenac eyedrops in preventing postoperative inflammation and long-term cystoid macular edema. J Cataract Refractive Surg. 1997;23/8:1183-1189. 4. Avitabile T et al. The effects of diclofenac on cystoid macular edema following cataract surgery. Invest Ophthalmol Vis Sci. 1995;36(4):S136. 5. Miyake K et al. Preventative effects of diclofenac ophthalmic solution on post-cataract cystoid macular edema. Acta Soc Ophthalmol Jpn. 1998;102/8:522-530. 6. Umezawa S and Shimizu K. Anti-inflammatory therapy following implantation of silicon intraocular lens. Jpn J Clin Ophthalmol. 1994;48/6:1271-1275. 7. Miyake K et al. Latanoprost accelerates disruption of the blood-aqueous barrier and the incidence of angiographic cystoid macular edema in early postoperative pseudophakias. Arch Ophthalmol. 1999;117/1:34-40. 8. Rho D. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg 2003;29:2378-2384. 9. Rho D and Soll S. Combination therapy for pseudophakic cystoid macular edema: diclofenac sodium 0.1% and prednisolone acetate 1% versus ketorolac tromethamine 0.5% and prednisolone acetate 1%. ARVO abstract 2004.
|
|
Pre-existing ocular and systemic conditions can place patients at risk of post-cataract extraction CME |
Ocular Predisposing Factors
Perioperative Factors
Ocular Inflammatory Conditions
Systemic Predisposing Factors
|
REFERENCES
1. Fu A, Ahmed I, Ai E. Cystoid macular edema. In Ophthalmology, Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 956-962.
2. Rossetti L, Chaudhuri J, Dickersin K. Medical prophylaxis and treatment of cystoid macular edema after cataract surgery. The results of a meta-analysis. Ophthalmology. 1998;105:397-405.
3. Arshinoff SA, Opalinski YAV. The pharmacotherapy of cataract surgery. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 331-336.
4. Zimmerman PL, Boyle TM. Pars planitis and other intermediate uveitis. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 1213-1218.
5. Goodman DF, Stark WJ, Gottsch JD. Complications of cataract extraction with intraocular lens implantation. Ophthalmic Surg. 1989;20(2):132-140.
6. Ray S, D'Amico DJ. Pseudophakic cystoid macular edema. Semin Ophthalmol. 2002;17(3-4):167-80.
7. Kohnen T, Wang L, Friedman HJ, et al. Complications of cataract surgery. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 381-390.
8. Roberts CW. The prophylaxis of cystoid macular edema following cataract surgery. Ther Updates Ophthalmol. Special Issue 2003:5,7-9.
9. Forster DJ. General approach to the uveitis patient and treatment strategies. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 1115-1120.
10. Cowen CL. Sarcoidosis. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 1185-1190.
11. Guex-Crosier Y. The pathogenesis and clinical presentation of macular edema in inflammatory diseases. Doc Ophthalmol. 1999;97(3-4):297-309.
12. Kempen JH, O'Colmain BJ, Leske MC, et al. The prevalence of diabetic retinopathy among adults in the United States. Arch Ophthalmol. 2004;122(4):552-563.
13. Zaczek A, Olivestedt G, Zetterstrom C. Visual outcome after phacoemulsification and IOL implantation in diabetic patients. Br J Ophthalmol. 1999;83:1036-1041.
14. Morley MG, Heier JS. Venous obstructive disease of the retina. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 864-871.
15. Jain R, Stevens JD, Bunce CV, et al. Ischaemic heart disease may predispose to pseudophakic cystoid macular edema. Eye. 2001;15:34-38.
16. Sieving SA. Retinitis pigmentosa and related disorders. In Ophthalmology. Yanoff M, Duker JS, eds. 2004, Mosby: St. Louis, MO. p. 813-823.
17. Grant WM, Schuman JS. Toxicology of the eye: effects on the eyes and visual system from chemicals, drugs, metals and minerals, plants, toxins, and venoms; also systemic side effects from eye medications. 4th ed. Springfield, IL: Charles C Thomas; 1993; 629-640, 1040-1041.
18. Miyake K, Ibaraki N. Prostaglandins and cystoid macular edema. Surv Ophthalmol. 2002;47(Suppl 1):S203-218.
19. Solomon KD, Cheetham JK, DeGryse R, et al. Topical ketorolac tromethamine 0.5% ophthalmic solution in ocular inflammation after cataract surgery. Ophthalmology. 2001;108(2):331-337.
20. Flach AJ, Jampol LM, Weinberg D, et al. Improvement in visual acuity in chronic aphakic and pseudophakic cystoid macular edema after treatment with topical 0.5% ketorolac tromethamine. Am J Ophthalmol. 1991;112(5):514-519.
21. Flach AJ, Stegman RC, Graham J, et al. Prophylaxis of aphakic cystoid macular edema without corticosteroids. A paired-comparison, placebo-controlled double-masked study. Ophthalmology. 1990;97(10):1253-1258.
22. Rho DS. Treatment of acute pseudophakic cystoid macular edema: Diclofenac versus ketorolac. J Cataract Refract Surg. 2003;29(12):2378-2384.
23. Heier JS, Topping TM, Baumann W, et al. Ketorolac versus prednisolone versus combination therapy in the treatment of acute pseudophakic cystoid macular edema. Ophthalmology. 2000;107(11):2034-2038;discussion 2039.