new
technology user guide: oct
Case Studies and Practice Patterns
Illustrate the Utility of OCT in a Comprehensive Practice
BY DAVID J. PALMER, M.D.
In my comprehensive practice with a glaucoma subspecialty, we see glaucoma suspects who are referred to us for further evaluation and patients who are already diagnosed with glaucoma to determine if surgical intervention is necessary.
Optical coherence tomography (OCT) is one of the tools we use in examining our patients and to guide our treatment decisions. My practice includes retinal specialists who also use OCT as an examination tool.
In this article, I discuss how we use the StratusOCT in glaucoma testing and present case studies to illustrate its value.
Early Detection is Important
Glaucoma, as you know, is a multifactorial condition that results in retinal ganglion cell (RGC) loss and a progressive optic neuropathy. These structural changes may precede visual changes. In the Ocular Hypertensive Treatment Study (OHTS), >50% of enrolled patients who developed glaucoma had structural optic nerve changes prior to visual field loss. Quigley and colleagues demonstrated that optic nerve head (ONH) abnormalities, RGC death, and defects in the retinal nerve fiber layer (RNFL) appear prior to visual field loss. Specifically, 35% to 50% of the optic disc is damaged before visual field changes occur. One study has shown that ONH structure correlates with visual function as visual field defects occur in areas with thinner RNFL and neural rims. So early and accurate assessment of visual function is essential.
The StratusOCT Instrument
As ophthalmologists, we use various means to assess a patient's visual function: achromatic automatic perimetry (AAP), short wave automated perimetry (SWAP), frequency doubling technology (FDT), and multifocal visual evoked potential (mVEP). Optic nerve and RNFL structural measurement techniques include stereo disc photography, red-free RNFL photography, confocal scanning laser ophthalmoscopy (HRT II), scanning laser polarimetry (GDx), and OCT. Our goal is to diagnose glaucoma pre-perimetrically to prevent visual function deterioration.
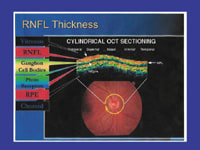
The StratusOCT, manufactured by Carl Zeiss Meditec, is a scanning interferometer that uses high-resolution (+/-8 to 10 microns) to obtain cross-sectional images of the retina, based on the reflectivity of different tissue layers within the retina. Similar to A-mode ultrasound, the superluminescent diode light source scans transversely across the eye to measure tissue thickness.
For glaucoma, circular scans around the ONH and linear scans across the ONH have proven most valuable. Macular thickness correlation with glaucoma is under investigation. OCT measurement reproducibility, agreement with histopathology and glaucomatous visual fields, and comparisons with other scanning laser devices have demonstrated good correlation.
|
|
|
|
OCT scan correspondence to fundus
image. |
|
How the Technology Fits Into My Patient Management Protocols
We order baseline StratusOCT examinations for all patients with normal visual fields, asymmetric disc cupping, normal or elevated IOPs, monocular status, and risk factors such as thin corneal measurements, positive family history of glaucoma, and African-American race. By doing the OCT examinations ourselves, we save referral time and costs and add to the patient's convenience.
A typical examination includes vision and pupil testing, confrontation fields, and slit-lamp biomicroscopy. We especially note any media opacities that may interfere with OCT image resolution. We perform applanation tensions, gonioscopy, dilation with stereoscopic disc viewing, corneal pachymetry, and a Humphrey Sita-Standard 24-2 VF test (HVF) and review any additional documents and photos. If a patient's FDT, HRT, or GDx is inconsistent with clinical findings and an HVF, then we perform an OCT scan. I prefer the Fast RNFL strategy because results parallel the Regular RNFL test. I also routinely obtain baseline stereo disc photographs to eliminate the need for an OCT disc analysis. If the macular function test establishes cystoid macular edema as the etiology of vision loss in patients on, for example, prostaglandin agents, we would make a medication adjustment.
An experienced technician performs each OCT scan by accurately placing the reference circle around the ONH and qualitatively selecting the best scan patterns for analysis. Off-centered circle placement will yield RNFL readings thinner farther from and thicker closer to the ONH, potentially creating inter-test measurement discrepancies. The scans can be seamlessly incorporated as a part of the initial work-up while the patient is dilating. Once analyzed, the printout displays the TSNIT pattern for each eye, compares patient data with an age-related normal population, and includes a photo of the disc with the reference circle placement. We often initiate medication in high-risk patients with pre-perimetric RNFL attenuation.
We use comparative analysis software to interpret multiple scans, and we adjust target IOPs according to test outcomes. If a patient is on maximum-tolerated medical therapy and has a newly defined progression in the RNFL or ONH despite the lack of perimetric advancement, I may advocate a laser trabeculoplasty to further reduce ocular tension. I don't rely on the OCT results alone for any decision to perform incisional glaucoma surgery. Instead, I use the functional vision changes plotted on the Humphrey Visual Field Analyzer or structural changes observed on serial disc photo comparisons, confirmed as necessary by the StratusOCT. We also believe that surgery should be reserved to stabilize visual deficits that will potentially interfere with a patient's lifestyle.
Testing Frequency
The goal of any diagnostic test is to differentiate patients with normal IOP who have glaucoma; patients with elevated IOP who have glaucoma; and patients with elevated IOP who are suspects only. The StratusOCT test is most useful for pre-perimetric or early glaucoma. We rely on HVF defect progression for moderate to advanced glaucoma stages. We typically repeat the OCT tests annually and retest the HVF more frequently if there are any new suspicious changes or further RNFL thinning is indicated by the OCT.
Case Studies
These cases from my practice illustrate the situations in which we find the StratusOCT to be most useful.
Case 1. 52 y/o female with LTG for 10 years. Past IOPs peaked at 20 mmHg and are usually 9 to 13 mmHg on medications. Corneal thickness measures 560 to 565 microns OU. C/Ds are OD 0.75, OS 0.55 to 0.6. The HVF shows changes nasally OD with a possible SNS change OS. The OCT pattern is consistent with the HVF OD and reflects definitive RNFL thinning OS, indicating the need for bilateral glaucoma therapy.
Case 2. 72 y/o male with COAG OD with peak IOPs to 25 mmHg and tensions in the low-mid teens on treatment. CT is 565 OD, 590 OS with a C/D OD of 0.55V x 0.25H and OS 0.15-0.2. HVF abnormalities are consistent with the increased vertical disc cupping on the right, confirmed by OCT testing. No testing abnormalities were evident OS, and subsequent examinations demonstrate a stable course.
|
|
|
|
Based on the StratusOCT and HVF results, we established a new target IOP goal for this patient with frequent pressure and HVF
monitoring. |
Case 3. 74 y/o female with pseudoexfoliation (PXF) OD and IOPs to the mid-20s off medications. On medications, IOPs are in the upper teens. CT is 610 to 619 OU with a C/D of about 0.3 OU. Asteroid hyalosis was present bilaterally. Based on the normal HVF and OCT results combined with thicker corneas, medications were discontinued and patient was advised she did not have glaucoma. Vitreous opacities did not influence the OCT testing.
Case 4. 44 y/o female with COAG OD with IOPs untreated to 24, and treated IOPs to 13 to15 OU. CT OD is 500, OS 510 microns. C/D OD is 0.9H x 0.8V, OS 0.75V x 0.8H. Superior HVF changes OD progressed on both the HVF and OCT tests. HVF OS is normal and consistent with the OCT. Established a new target IOP goal of <13 mmHg with more frequent pressure and HVF monitoring.
Case 5. 83 y/o pseudophakic male glaucoma suspect. IOPs in the mid-teens, thin CTs between 515-520 microns OU, and small C/Ds of 0.15-0.2. An inferior conus OS was responsible for the HVF defect confirmed by the thinner inferior RNFL on OCT testing. Patient's ocular status remains stable with observation only.
Case 6. 55 y/o male with LTG and peak IOPs to 18 mmHg. On medications, tensions are usually 12 to 14 mmHg. The CT OD is 550 and OS 575 microns, with a C/D OD of 0.8 and OS 0.6 to 0.65. HVF is abnormal but the OCT printout interpretation is normal. Closer inspection of the TSNIT pattern revealed a localized supertemporal RNFL thinning matching the inferonasal HVF depression. Serial OCT scans and vision function tests continue on current medications.
Case 7. 57 y/o male with PXG OD. Endstage CNAG OS (NLP), IOP OD 15, and OS 52 mmHg on medications, with a CT OD 542 and OS 620 microns. C/D OD was 0.4. A mature cataract prevented a posterior OS view. HVF was normal, but the OCT registered thinning of the RNFL superotemporally. Treatment was continued.
Case 8. 83 y/o male with COAG and IOPs in the mid-upper teens and average CT measurements. C/Ds of 0.2 OU and disc staphylomata. OCT could not be interpreted due to marked artifact. No treatment was initiated and patient's course is unchanged.
Case 9. 47 y/o female with 0.75 C/Ds OU and tensions in the upper teens. Presented with abnormal FDT tests, OS worse than OD. HVF results were unremarkable OU, and OCT RNFL measurements were normal for age. Patient was reassured that increased disc cupping was likely physiologic, and FDT results were probably inaccurate. Scheduled a follow-up visit in 1 year to reassess.
Documentation
The treating physician is responsible for documenting the purpose for the test, results of the test, interpretation of results on a separate, signed report, and discussing the results with the patient. The report date, technician's signature, and clinical implications are compulsory. The progress note always includes comments on changes in medical therapy and follow-up procedures.
Cost Effectiveness
The StratusOCT, CPT Code 92135, is billed per eye and is normally reimbursed when performed on an annual basis. Some insurance companies, however, do not recognize the benefits of scanning laser diagnostic imaging technology despite supportive evidence from the medical literature. The cost effectiveness of the procedure is calculated based on the instrument purchase price, extended warranty plan, reimbursement rate, number of ocular scans performed, and tax considerations. Sharing costs with other associates or specialties can reduce expenditures. Consultation with a StratusOCT company representative and perhaps a tax advisor will help determine purchase or leasing feasibility.
If your patient volume is suboptimal for instrument acquisition, then centers such as academic institutions may offer the test as an outpatient service with an interpretation of the results.
Benefits of the StratusOCT
The StratusOCT offers several major benefits to practices with a glaucoma specialty.
First, as a totally objective measure, the OCT doesn't require the patient to do anything; in other words, there are no buttons the patient has to push. Other benefits are:
- useful in diagnosing glaucoma with mild to moderate macular dystrophy and good target fixation associated with inaccurate HVF testing
- provides a 3D analysis
- differentiates congenital disc enlargement from glaucomatous loss
- differentiates disc drusen vs. glaucoma HVF loss
- useful in deciding whether to implement more or less aggressive glaucoma therapy based on nerve layer thickness
- helps clarify data if data reports conflict
- correlates monocular patient C/D-RNFL
- does not have an arbitrary reference plane or corneal polarization influence
- tissue reflectivity helps define tissue type.
There are, however, a few limitations:
- denser media opacities obtained can result in unreliable readings
- a patient who can't sit still for a period of time or has poor head positioning can affect results
- produces artificial results for peripapillary staphylomata -- you get a flat line
- no eye tracker provided for saccadic movements
- the normative database is limited to 328 patients
- the reference circle has to be placed manually
- the instrument can't be used with a pupil size <3mm.
Dr. Palmer is in private practice in the Chicago area. He is clinical assistant professor at Northwestern University and a committee member of the American Academy of Ophthalmology's EyeCare America/Senior EyeCare program and the Illinois Association of Ophthalmology. Dr. Palmer is not affiliated with Carl Zeiss Meditec.
REFERENCES
1. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertensive Treatment Study. A randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open angle glaucoma. Arch
Ophthalmol. 2002; 120:701-713.
2. Quigley HA, Addicks EM, Green WR. Optic nerve damage in human glaucoma. III. Quantitative correlation of nerve fiber loss and visual field defect in glaucoma, ischemic neuropathy, papilledema, and toxic neuropathy. Arch Ophthalmol. 1982; 100:135-146.
3. Zeyen TG, Caprioli J. Progression of disc and field damage in early glaucoma. Arch Ophthalmol.. 1993; 111:62-65.
4. Caprioli J, Miller JM. Correlation of structure and function in glaucoma. Quantitative measurements of disc and field. Ophthalmol. 1988; 95:723-727.
5. Guedes V, Schuman J, Hertzmark E, et al. Optical coherence tomography measurement of macular and nerve fiber layer thickness in normal and glaucomatous eyes. Ophthalmol. 2003; 110:177-189.
6. Jaffe G, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004; 137:156-169.
Case Studies and Practice Patterns
Illustrate the Utility of OCT in a Retina Practice
BY JEEVAN R. MATHURA, JR., M. D.
Optical coherence tomography (OCT) is a valuable addition to fluorescein angiography (FA) in the diagnosis and management of many retinal diseases. It's noninvasive, requiring no dye injection, which is especially useful in patients who are allergic to fluorescein. It's relatively easy to obtain good quality images, and it can be performed more quickly.
Used separately, both FA and OCT provide useful information about the pathology in the retina and vitreous interface. Together, they complement each other -- the OCT images a cross section of the retina, and combined with the FA, you can image the retina in 3 dimensions. OCT is now being used as an adjunct to FA in multicenter clinical trials and in the treatment of diabetic macular edema (DME) and choroidal neovascularization (CNV).
In my retina practice, I regularly use the StratusOCT to evaluate patients with DME, CNV, and macular degeneration. I find it makes my job easier in explaining to patients how their vision is being affected and what we as clinicians can do about it.
In this article I describe how I use the StratusOCT in my practice and present case studies that illustrate its clinical value in diagnosing retina disorders.
How the Technology Fits Into My Practice
I do a StratusOCT when I detect signs of progressing macular degeneration and diabetic retinopathy. It's especially useful in the preoperative evaluation of cataract patients with a history of diabetes or macular degeneration because the StratusOCT can image abnormalities of the macula, such as retinal thickening, even when the view with biomicroscopy is limited.
Often I get patient referrals after they've had an OCT elsewhere, and I don't repeat the test to verify results. But if some intervention has been performed, or if there has been some change in visual acuity or fundus examination since the time of referral, then I usually repeat the OCT.
The OCT is useful in diagnosing and staging macular holes, detecting traction on the macula, and imaging the epiretinal membrane (ERM). I also use the OCT to rule out or measure thickening in the macula, when a patient complains of decreased vision and there's no obvious thickening in the macula, or I can't find an explanation for the decreased vision.
You can use the StratusOCT in 2 different modes, the line scan mode and the fast macular scan. I always get the line scan to give me the highest resolution image. I will also get a fast macular scan if I want to quantify retinal volume, and this is especially useful with patients who have difficulty maintaining fixation for the 1 or 2 seconds required to do the line scan.
The StratusOCT Helps With Difficult Examinations
The StratusOCT is used in examining patients who are difficult to examine, whether it's poor cooperation, poor dilation, or media opacity. For example, it can be difficult to get a stereoscopic view of the retina in a patient with a poorly dilated pupil to assess the macula for thickening. Also, unclear media such as cataract or asteroid hyalosis may be an impediment to getting good visualization with biomicroscopy of thickening in the macula. The StratusOCT can image the macula through a small pupil, and because it acquires images quickly, it's useful with patients who are unable to maintain fixation. It's even possible to image patients with nystagmus.
|
|
|
|
A patient with AMD, high-risk
drusen, and decreased vision after cataract surgery. |
|
OCT Can Support CNV Diagnoses
Fluorescein angiography is still the gold standard for diagnosing CNV. However, an OCT can support the diagnosis, as the following case studies show.
OCT is also a useful application for cataract patients or the postoperative patient who has decreased vision as Irvine-Gass syndrome cannot always be assumed.
A 75 y/o woman with a history of Fuch's dystrophy had significant corneal edema after cataract surgery. Visual acuity was 20/300. The edematous cornea limited any view of the retina with slit lamp biomicroscopy. To determine whether keratoplasty was indicated, an OCT was performed to assess the retina. The scan showed subretinal fluid indicative of CNV, which was confirmed with an FA.
A 72 y/o man had decreased vision after cataract surgery. Initially, his best corrected visual acuity was 20/25, but it soon declined to 20/40. He was treated with topical ketorolac tromethamine and prednisolone acetate for presumed Irvine-Gass syndrome. After 3 months on topical treatment and with no improvement, an OCT was done that showed a fibrovascular pigment epithelial detachment. The presence of this form of occult CNV was confirmed with an FA and an ICG angiogram. The patient was given a posterior sub-Tenon's injection of 40 mg triamcinolone acetonide. The subretinal fluid resolved and his vision returned to 20/25.
The presence of subretinal fluid or intraretinal thickening can be helpful in deciding when to treat CNV with an equivocal fluorescein. The StratusOCT can easily detect fluid in the retina, under the retina, and under the retinal pigmented epithelium (RPE). It can also image through hemorrhage in or under the retina. The StratusOCT is also invaluable in ruling out CNV in patients with high-risk drusen.
A 64 y/o man with a history of krypton laser for presumed central serous chorioretinopathy 12 years earlier who noted severe vision loss in the same eye of counting fingers acuity. On ophthalmoscopy, a large area of subretinal and sub-RPE hemorrhage was noted. The OCT demonstrated large serosanguinous pigment epithelium detachments that were suspicious for idiopathic polypoidal choroidal vasculopathy (IPCV). An ICG angiogram revealed multiple "hot spots" around the disc and in the fovea, consistent with the diagnosis of IPCV, and the patient was treated with photodynamic therapy with verteporfin.
A 72 y/o man has a history of nonexudative AMD with high-risk drusen in 1 eye. The visual acuity in the other eye is 20/400 from CNV and subsequent disciform scar formation. The patient was very concerned about losing vision in his better eye. Subsequently, after an examination showed no evidence of CNV in his better eye, he complained of a change in his vision. He had been monitoring the Amsler grid and thought he had noticed increasing metamorphopsia. His visual acuity was unchanged. On ophthalmoscopy, there was no hemorrhage or obvious thickening of the retina. To confirm there was no CNV, an OCT was done. It showed no thickening of the retina or fluid under the retina or RPE. The OCT reassured both the patient and the clinician, and the risk of a needle stick or a dye injection was averted.
Using OCT to Diagnose and Treat DME
As most retinal specialists know, diabetic retinopathy is the leading cause of vision loss in working-age Americans, and macular edema is the most common cause for vision loss in this population. Early detection, therefore, is important to preserve vision. The OCT can assist in the diagnosis and treatment of DME.
Studies suggest that an OCT is more than sensitive for detecting DME than slit-lamp biomicroscopy of the macula, the current standard of care. The OCT also assists in educating patients about their eye condition because they can see the images of their retina and then understand why they need treatment. Education is especially useful in a young diabetic patient that has clinically significant macular edema from diabetes but still has good vision.
Once the decision to treat is made, the OCT can help guide me as to where to treat, although FA is the current standard of care. By using the retinal thickness map, which shows where the retina is thickest and where the laser should be applied.OCT also helps me to assess the effectiveness of treatment. Postoperative cystoid macular edema also is easily diagnosed with the StratusOCT.
A 50 y/o woman has thickening in the macula from diabetic macular edema. Visual acuity in one eye is 20/25. The retinal thickness map shows that most of the thickening is inferior to the macula. The OCT helped convince the patient that something was wrong with her retina by showing the abnormal thickening of the retina. Focal laser was applied to the inferior macula, which stabilized her vision.
|
|
|
|
A line scan showing cystoid spaces in the retina consistent with
CME. |
A 67 y/o woman had cataract surgery 6 months prior with vitreous loss during the procedure. The patient had a history of dry macular degeneration with high-risk drusen. The best-corrected visual acuity was 20/25. Acuity recently dropped to 20/40, and the patient was referred for a retinal examination to rule out CNV. The OCT showed cystoid spaces in the retina, and no subretinal fluid was noted indicating cystoid macular edema, which was confirmed by the FA. Patient was treated with a topical nonsteroidal antiinflammatory drug (NSAID).
The StratusOCT can aid in the diagnosis and management of macular edema caused by retinal vein occlusions, similar to diabetic macular edema, by quantifying the amount of macular edema from the vein occlusion and monitoring the response to treatment.
A 42 y/o man with type 2 diabetes and hypertension had a nonischemic central retinal vein occlusion. Visual acuity was 20/200. The OCT showed large intraretinal cystoid spaces and subretinal fluid. The patient was given an intravitreal injection of triamcinolone acetonide. Two weeks later his visual acuity improved only to 20/80, but the CME had improved significantly. He was reassured that he should have more improvement in his vision, and 6 weeks after injection, his acuity improved to 20/50.
Disorders of the Vitreoretinal Interface
Diagnosing disorders of the vitreoretinal interface can be difficult by biomicroscopy or FA as some of the entities may be difficult to see or they may have a similar appearance. The OCT affords an easy diagnosis. For example, vitreomacular traction syndrome is very difficult to see with a slit lamp. Although a biomicroscopy may show traction and an FA may show hyperfluorescence in the macula, the OCT images a cross-section of the vitreomacular interface, which becomes not only an easy diagnosis but also a reliable one.
|
|
|
|
The OCT can easily image traction on the
fovea. |
|
The StratusOCT can easily distinguish full-thickness macular holes from lamellar holes and pseudoholes. ERM too is often easy to diagnose with biomicroscopy; however, it might be difficult to determine if it's very adherent to the retina or if there's associated macular edema.
A 66 y/o woman was referred for reported distortion in one eye. Distortion was reproducible with Amsler grid testing but ophthalmoscopy was unremarkable, except for the absence of a foveal reflex. However, the OCT easily imaged the traction on the fovea.
A 70 y/o man noticed blurred vision and distortion in one eye, which had a visual acuity of 20/60. The OCT revealed a full thickness macular hole, and the patient was referred for pars plana vitrectomy.
A 63 y/o woman had a complicated cataract surgery with rupture of the posterior capsule and vitreous loss. Postoperatively, the patient complained of monocular diplopia and micropsia. Visual acuity was 20/70. An OCT revealed an ERM with associated macular edema. Before a surgical intervention was considered, a series of 2 posterior sub-Tenon's injections of triamcinolone acetonide was given, which resulted in resolution of the macular edema and improvement of visual acuity to 20/30. The patient also reported resolution of the diplopia and micropsia.
A 55 y/o man had gradual worsening of his visual acuity after cataract surgery to 20/70. A very obvious epiretinal membrane was noted on biomicroscopy. The OCT confirmed the epiretinal membrane and showed there was no associated macular edema. He was referred for pars plana vitrectomy and membrane peeling.
Cost Effectiveness
Besides being an excellent patient education tool, the StratusOCT for me is a timesaver and in the long run a cost saver. An OCT picture is worth a thousand words when trying to explain macular pathology to a patient because, for example, the hole in the retina as shown on the OCT can be easily seen, and the patient can more quickly understand what I am explaining. This saves me time.
As another example, when I see a patient that has macular pathology, I tell that patient I want to a quick scan of the back of the eye to give me a better idea of what's going on. While the patient is getting the OCT, I can move on to the next patient. When the scan for the first patient is ready, the technician will either put the image on the monitor in the examination room or present the printout. I then explain to the patient what's going on more effectively.
Benefits and Limitations of the StratusOCT
The StratusOCT is an excellent tool to assess the retinal architecture in the macula quickly and easily, and it can often make the diagnosis in a difficult case. I have given many of the specific benefits in the above case studies.
However, the most important limitation is the StratusOCT doesn't directly assess retinal circulation, so it can't be used to rule out retinal ischemia in diabetic retinopathy, retinal vein and artery occlusions, and ocular eschemic syndrome. For these situations, an FA is better.
Dr. Mathura is a medical retina specialist. He is an assistant professor of ophthalmology at Northwestern University Feinberg School of Medicine, Chicago, Ill. He is not affiliated with Carl Zeiss Meditec.
Acknowledgement: Images were provided by Jonathan B. Shankle, CRA.
REFERENCES
1. Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. II. Prevalence and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Arch
Ophthalmol. 1984;102:520-526.
2. Browning DJ, McOwen MD, Bowen RM Jr, O'Marah TL. Comparison of the clinical diagnosis of diabetic macular edema with diagnosis by optical coherence tomography. Ophthalmology. 2004;111(4):712-5.
3. Nguyen QD, Shah SM, Van Anden E, Sung JU, Vitale S, Campochiaro PA. Supplemental Oxygen Improves Diabetic Macular Edema: A Pilot Study. Invest. Ophthalmol Vis Sci 2004;45: 617-624.
4. Browning DJ. Potential pitfalls from variable optical coherence tomograph displays in managing diabetic macular edema. Am J Ophthalmol. 2003;136(3):555-7.
5. Browning DJ, Fraser CM. Optical coherence tomography to detect macular edema in the presence of asteroid hyalosis. Am J Ophthalmol. 2004;137(5):959-61.
6. Mori K, Gehlbach PL, Sano A, Deguchi T, Yoneya S. Comparison of epiretinal membranes of differing pathogenesis using optical coherence tomography. Retina. 2004;24(1):57-62.
7. Voo I, Mavrofrides EC, Puliafito CA. Clinical applications of optical coherence tomography for the diagnosis and management of macular diseases. Ophthalmol Clin North Am. 2004;17(1):21-31.