A Guide to Effective Use of Glaucoma Imaging
We've found that these protocols produce the best clinical and practice management results.
BY ROBERT J. NOECKER, M.D., M.B.A.
In our practice, we've found that effective use of glaucoma imaging starts with the first visit. In this article, I'll explain how we use imaging as an integral tool in monitoring and managing glaucoma and glaucoma-suspect patients over a period of time.
When first seeing a glaucoma suspect or glaucoma patient, we obtain good baseline data with which to compare future results. Our technicians begin by doing a Humphrey 24-2 SITA visual field. Then I perform an anterior segment exam, including gonioscopy. The technician does pachymetry and then dilates the patient for imaging. Whether you're imaging with the Stratus OCT, HRT II, GDxVCC, or RTA, these points will help you to obtain the best patient management and practice management results:
The gold standard is still fundus photographs. Preferably these should be stereo disc photos. They should be repeated every 3 to 5 years, unless an abnormality like an optic nerve hemorrhage is present when the patient comes in for an exam. In those cases, we take a photograph so that we can correlate it with future optic nerve changes and visual field defects.
If possible, we take care of this on the patient's first visit. However, it's usually not feasible to bill for both imaging and fundus photography at the same visit, so we may defer the photography until a later visit. I prefer not to postpone imaging because it often provides more information immediately.
Do visual field testing before digital imaging. Imaging tests sometimes require dilation. Many dilating and anesthetic drops can cause drying and blurring of the ocular surface, affecting visual field results.
Check corneal thickness. Patients with thinner corneas are in a higher-risk group than those who have thicker corneas.
Compare data. If imaging and visual field results don't agree with each other, how I react depends on the nature of the disagreement:
- If imaging shows that the nerve fiber layer (NFL) is thin in one area but the white-on-white Humphrey field is normal, I may have the patient come back in a month and do a different type of field, such as an FDT, FDT Matrix, or SWAP perimetry. Although some of these tests are more difficult to do, they're more sensitive than early white-on-white visual field testing.
- If I find a visual field defect but the imaging doesn't correlate, I try imaging using a different technology. If you don't have access to another imaging technology, I recommend having the patient come back to repeat the test sooner than you otherwise might -- perhaps in a couple of months, rather than in a year.
When and How We Test
If the patient is healthy and not progressing, performing each test once a year is our standard.
We have the patient come in every 6 months: At one visit we perform a visual field; at the next we perform imaging. However, if the patient has very thin corneas or borderline test results, we consider him higher-risk and may have him come in every 3 months instead of every 6 months initially, especially if he has a high-risk family history or other risk factors.
If a patient comes in for his annual visual field and the results look suspicious, we have him come back for imaging, or even do it the same day. Visual field testing can be affected by many factors, so we wouldn't treat solely on the basis on one suspicious visual field.
Naturally, if we know that visual field deterioration is occurring, we intervene to lower IOP and compress the intervals between visits. Once the patient has stabilized, we stretch the timing out again. If it's a small change, we'll have the patient return in 3 to 6 months to re-establish a baseline. If it's a big change, we'll usually see the patient more frequently until we're sure the pressure has been adequately reduced.
Be Aware of These Factors
Other factors that can impact the effective use of imaging for glaucoma include:
Who does the testing? Imaging is nearly always done by technical staff, but because testing can be tedious, many offices delegate it to the most inexperienced person. This is usually a bad idea. If the technician forgets a detail, fails to manage the patient effectively or doesn't realize that something is happening that will affect the outcome, he or she can waste 20 minutes and produce useless results. For example, when using the HRT II, the person performing the test must draw a line around the rim of the optic nerve, and all subsequent tests are based on where the rim marking is initially placed. Someone who's inexperienced or inconsistent can cause a long-lasting problem.
Billing issues. The technician can document that the test was done, but the doctor is responsible for the professional component, which must include some kind of quantitative interpretation. This can be as simple as recording the mean NFL thickness and "no change from prior exam on XX date," or "thinning of NFL at 6 o'clock correlates with superior arcuate defect," but it has to be there. You can't just sign your initials. The same holds true for pachymetry; you need to document an interpretation of the resulting data.
Office layout. Group the testing instruments where your glaucoma patients are seen. The time a technician spends escorting the patient to a test can be significant. The one exception might be the fundus camera, which is generally used less frequently.
Robert J. Noecker, M.D., M.B.A., is associate professor of ophthalmology, vice chairman for clinical activities, and director, glaucoma service, at the University of Pittsburgh Medical Center Eye and Ear Institute. He can be reached via e-mail at noeckerrj@upmc.edu.
Obtain Quality Anterior Segment Photos with a $500 Camera
BY JERRY HELZNER, SENIOR EDITOR
|
|
|
|
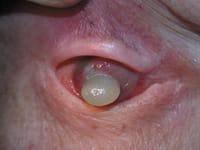
Patient with history of orbital squamous cell carcinoma presents with anterior tumor extension and crystalline lens extrusion. |
|
For an outlay of approximately $500 for a standard handheld digital camera, and using no specialized equipment, Jonathan M. Davidorf, M.D., says he's capturing high-quality digital anterior segment images that are comparable to those obtained using cameras costing 10 or 20 times that amount.
Dr. Davidorf, assistant clinical professor at UCLA's Jules Stein Eye Institute and director of Davidorf Eye Group in West Hills, Calif., uses a Canon Power Shot S45 model camera, but says the newer Canon Power Shot S50 will also do the job.
"To get good results, it's very important that the lens diameter on the camera be about the same as the diameter of the ocular on your slit lamp," advises Dr. Davidorf. "But finding a camera with a lens that matches the slit lamp shouldn't be a problem."
Dr. Davidorf's basic shooting technique is to position the camera at one of the oculars of the slit lamp and focus on a specific portion of the anterior segment.
"Just use the slit lamp normally and set the camera to 'macro mode' with the flash turned off," he says.
|
|
|
|
Following penetrating keratoplasty, a double anterior chamber can be seen due to retained recipient Descemet's/endothelium. |
To photograph sclerotic scatter, Dr. Davidorf places the slit lamp on a low-power setting and has an assistant focus a light source, such as a muscle light, on the sclera.
"For the most part, I've been putting the photos into the computer and using them for research and in presentations," says Dr. Davidorf. "But they can also be helpful in monitoring pterygiums, herpes, corneal ulcers and anterior segment tumors. With phakic IOLs, I'll use these photos for follow-up after implantation to check for lens opacities or changes in the vault."
Dr. Davidorf favors using the standard digital camera because he knows immediately if he has taken a good-quality photo.
"If it doesn't come out right, I can take the photo again," he notes. "There's no waiting to see the result."
Looking ahead, Dr. Davidorf believes that adding an adapter and footswitch could enable a standard handheld digital camera to do high-quality fundus photography.
"I see no particular reason why that couldn't be done," he concludes.