at press time
Alcon Suffers Setback with AMD Drug
Retaane Fails to Show Superiority to PDT in Trial.
Alcon's expectations of developing a clearly superior treatment for wet AMD were dealt a major blow when the company announced recently that its anecortave acetate (Retaane 15 mg Depot) treatment for wet AMD failed to demonstrate superiority to Visudyne in a large Phase III comparative trial.
|
|
|
|
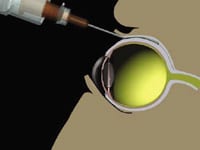
To stop reflux, Alcon has added a counter-pressure device (not shown) to the existing Retaane delivery
system. |
|
In a conference call following the announcement, Alcon Senior Vice President, Research & Development, Gerald Cagle, Ph.D., said he was "surprised and disappointed" that, at 12 months, only 45% of the 214 patients who had received Retaane showed stable or improved vision (loss of less than 3 lines). In a previous Phase II/III study, 73% of the patients who received Retaane showed stable or improved vision after 24 months. In this latest trial, 49% of the 220 patients who received Visudyne photodynamic therapy demonstrated stable or improved vision, a somewhat lower percentage than in previous trials involving Visudyne.
Despite the lackluster efficacy data, Alcon said Retaane continues to show an excellent safety profile. The company said it still has high hopes for the drug "as a viable and important tool for retinal specialists" and will file a New Drug Application for Retaane with the FDA later this year.
In attempting to explain the wide difference in efficacy shown in the two Retaane trials, Dr. Cagle said that reflux, or leakage of the drug, could be primarily responsible. Evidence of reflux means that not all of the drug was staying in its intended area, behind the macula.
Dr. Cagle said Alcon researchers had seen "some reflux" in the earlier clinical trial, but had concluded that it was a controllable factor that didn't have a significant influence on the efficacy of Retaane.
To correct the reflux problem, Alcon has designed what it calls "a simple counter-pressure device" that it has now incorporated into the delivery system. The FDA has asked the company to conduct a new clinical trial that uses the counter-pressure device. This trial is currently enrolling patients.
Dr. Cagle said the company is now looking into all possible variables that could account for the discrepancy in the trial results. In addition to the effect of reflux, these include differences in the patient population, and any variations in the training provided to investigators in administering the drug.
IntraLase Sells Shares to Public
Flap-Cutting Laser Draws Investor Interest.
IntraLase, the California technology company that developed the IntraLase FS femtosecond laser for cutting flaps in LASIK surgery, has received a warm reception from stock market investors.

Investors like the IntraLase story. When the company's stock made its debut on the NASDAQ market on Oct. 7 under the symbol ILSE, it shot up more than $3 a share from its offering price of $13, ending the day at $16.25 a share. The initial public offering of 6.6 million shares raised a total of $86.3 million, of which about $82 million went to the company to use in further developing its business.
Like many fledgling technology companies that go public, IntraLase isn't yet making a profit. For the 6 months ending June 30, the company reported a net loss of approximately $4 million on sales of $25.3 million. That compares with a loss of about $7 million on sales of $8.55 million for the same period a year ago.
According to IntraLase, surgeons using the FS femtosecond laser typically receive an average of $334 more per eye than those using a microkeratome. Also, a survey of surgeons found that the number of consultations resulting in surgery rises by 13% when patients are told their flap will be cut by the laser.
Analysts said one of the major attractions of IntraLase is that the company has no competitors in the area of flap-cutting lasers.
"They compete directly against the blades," said one analyst.
The IntraLase initial public stock offering was underwritten by a team of financial institutions led by Banc of America Securities.
VA Places Limits on
O.D. Surgery
AAO Isn't Satisfied with Supervision Requirement.
The Department of Veterans Affairs (VA) has announced a new policy requiring optometric laser surgery to be performed only under the supervision of an ophthalmologist. This VA limitation follows many months of debate on the issue and safety concerns raised by a coalition led by the American Academy of Ophthalmology (AAO), congressional leaders and veterans' service organizations.
"This directive represents a significant step by the VA to resolve a serious patient safety issue; however, the coalition does not believe that optometrists -- practitioners who do not attend medical school or fulfill surgical residencies -- have the proper training and education to perform invasive eye surgery," said Michael D. Maves, M.D., M.B.A., executive vice president for the American Medical Association.
The coalition has sought to work with VA leadership on resolving this veterans' safety issue, and will continue to do so in the weeks ahead, an AAO statement said.
"In light of VA's intention to proceed with this directive's approach despite our concerns, there remain several minimum level provisions that must be addressed before implementation to reduce the likelihood of a bad surgical outcome for veterans subject to optometric surgery," the statement continued. "These include establishing minimum optometric education and training standards and requiring direct ophthalmic supervision. In its current form, the directive does not adequately define supervision, so it could range anywhere from 'direct,' in which a U.S.-licensed ophthalmologist is in the operating room, to a mere reporting structure."
"We believe that an ophthalmologist-led team is the best way to ensure quality eye care for veterans, but allowing optometrists to perform invasive eye surgery -- even with ophthalmologic supervision -- lowers the bar and goes against the standard of care in 49 out of 50 states and in the U.S. military," said H. Dunbar Hoskins Jr., M.D., AAO executive vice president.
STAAR Doesn't Pass FDA Audit
Approval of ICL Phakic Lens Is Further Delayed.
With approval of its promising ICL phakic lens tied to the correction of manufacturing irregularities at its Monrovia, Calif., facility, STAAR Surgical was informed by the FDA in late September that further corrective action is needed.
|
|
|
|
FDA approval of STAAR Surgical's ICL phakic lens remains on
hold. |
|
The completion of an FDA audit undertaken at Monrovia in July resulted in 36 "observations" relating to various manufacturing processes at the Monrovia facility. And while some of the observations verified that adequate corrective action had been taken, other findings pointed out the need for further improvement.
Until the FDA is satisfied with the adequacy of the company's corrective action, it may take further steps. These could include conducting another inspection, seizure or recall of the company's products, injunction of the Monrovia facility to compel compliance, and/or suspension of production operations. In addition, the FDA is highly unlikely to approve the ICL until it's fully satisfied with the company's response in correcting its problems.
Delays in the approval of the ICL are becoming costly to STAAR, as Advanced Medical Optics recently received FDA approval to market the Verisyse phakic IOL, the first phakic lens to be approved for use in the United States. Until the FDA uncovered the manufacturing irregularities last December, STAAR had appeared on track to gain the "first mover" advantage in the U.S. phakic lens marketplace. Now, that advantage is lost.
Investors have become increasingly disenchanted with developments at STAAR as it became apparent that approval of the ICL would be delayed. From a high of more than $15 a share in the summer of 2003, STAAR's shares fell to a low of under $3 on Sept. 29, the day the FDA announced its audit findings.
In an apparently unrelated development, John R. Gilbert has resigned from STAAR's board of directors, citing personal reasons.
IN THE NEWS
Glaucoma medication. The FDA has issued an approvable letter for Alcon's Extravan ophthalmic solution for the treatment of glaucoma. Extravan solution is a fixed combination of travoprost 0.004% and timolol 0.5%.
"We will be meeting with the FDA as soon as possible to clarify what additional steps may be required to gain final approval to market Extravan," said an Alcon spokesperson.
Uveitis treatment. Bausch & Lomb says that in a clinical trial involving 239 patients, at 34 weeks its Retisert implant for the treatment of noninfectious posterior uveitis reduced the recurrence rate to 10% in treated eyes, as compared to a recurrence rate of 55.7% in untreated fellow eyes. In addition, about 21% of the treated eyes had an improvement in vision of 3 or more lines.
The Retisert implant combines proprietary drug delivery technology with the steroid fluocinolone acetonide.
Adverse events included cataract progression and increased IOP, which were anticipated given the nature of the disease and the type of drug used. These complications were managed by conventional means.
B&L says the new data essentially confirms the results of a previous clinical trial reported in 2003. The company anticipates commercialization of Retisert for posterior uveitis sometime next year.
OOSS executive director. The Outpatient Ophthalmic Surgery Society (OOSS) has named Claudia A. McDougal to the newly created position of executive director. McDougal has more than 20 years experience in association management, most recently as the CEO of the Metropolitan Denver Dental Society.
Patent dispute. Allergan has filed a patent infringement suit against Alcon in federal court relating to a proposed generic version of its Alphagan P glaucoma medication. Allergan said the suit was triggered by Alcon's application to the FDA to market a generic brimonidine tartrate ophthalmic solution 0.15% product in the United States.
"We are perfectly within our rights to market this generic drug, and we are going to pursue FDA approval for it," said Alcon spokesperson Mary Dulle.
AAO Helps Win Scope of Practice Victory
Optometry Interests Defeated in Puerto Rico.
The American Academy of Ophthalmology (AAO) joined forces with the Puerto Rico Ophthalmological Society to turn back the optometry lobby's legislative effort to dramatically expand optometric scope of practice. Ophthalmologists blocked legislation that would have allowed the broadest optometric scope of practice in the United States.
"If P.C. 4476 would have become law, Puerto Rico would have jumped from a jurisdiction with the most patient-friendly scope of practice to the broadest scope of practice in the United States, including current laws in Oklahoma," said Raul Franceschi, M.D., president of the Puerto Rico Ophthalmological Society. "This bill would have been unprecedented, and our eye M.D.s understood that they needed to act on behalf of patients."
The AAO said this was the latest in a growing number of national optometry-led initiatives to expand O.D.'s scope of practice. According to the AAO, the effort in Puerto Rico was the 46th attempt in 21 states by organized optometry in the past 7 years to legislate surgical privileges and the authority to perform injection procedures.
As introduced, P.C. 4476 would have allowed optometrists to prescribe any diagnostic, topical or oral drug, and enable them to perform injection procedures. In addition, the AAO said the bill could have provided a "blank check" to the optometry board to authorize optometrists to perform any surgery, including laser or cataract surgery. No state allows optometrists such a broad scope of practice, and the Academy saw this as an aggressive assault on safety.
After passing the Puerto Rico House of Representatives, the bill was stopped in the Senate. The AAO said this was due, in part, to the efforts of eye M.D.s to educate legislators on the patient risks associated with the bill.
"I hope every ophthalmologist takes note that we are battling optometry across the United States, and that their assaults on patient safety are not confined to specific state borders," said Cynthia Bradford, M.D., Academy secretary for State Affairs.
The Academy said it has helped defeat optometric scope of practice expansion legislation it considered detrimental to patient safety in five states this year.
IN THE NEWS
Vitrase as spreading agent. The FDA granted ISTA Pharmaceuticals 3 years of market exclusivity for hyaluronidase for injection; lyophilized, ovine (Vitrase) for use as a spreading agent to facilitate the dispersion and absorption of other drugs.
Vicente Anido, Jr., Ph.D., ISTA president and CEO, said "the approval of Vitrase earlier this year allowed for the reintroduction of a drug to the market that had previously been listed on the FDA's Drug Shortages list. To date, we have made the (bulk) product available to physicians free of charge. Once we have approval for the 150 USP units/mL vial, a smaller and more convenient configuration, we'll start full-scale commercialization efforts.
ISTA's Vitrase is a proprietary formulation of highly purified ovine hyaluronidase. In May, the FDA approved Vitrase in a 6,200 USP Units multipurpose vial. In August, ISTA filed a supplemental New Drug Application for a smaller, single-use, 150 USP Units/mL vial of Vitrase that the company believes will be more convenient and cost effective for use as a spreading agent. Pending FDA review and approval, ISTA plans to launch this new vial size in early 2005.
In addition, Vitrase has been studied for the treatment of vitreous hemorrhage and diabetic retinopathy. For additional information concerning the availability of Vitrase for use as a spreading agent, send an e-mail to Vitrase@istavision.com.
Major gift to Lighthouse. The Sol Goldman Charitable Trust has awarded Lighthouse International a gift of $10 million to name its headquarters building in Manhattan the "Sol and Lillian Goldman Building of Lighthouse International."
"We are thrilled to receive this wonderful commitment from the Sol Goldman Charitable Trust, the largest individual gift in our history, as a tremendous boost to our centennial celebration, which kicks off in April 2005," said Barbara Silverstone, DSW, president and CEO, Lighthouse International.
Sol Goldman was a major real estate developer who, at the time of his death in 1987, was second in New York City real estate holdings only to the city.