Education Forum Recap
Expanding Refrac tive
Horizons
Phakic IOLs are on the way. Here's how your patients with extreme myopia can benefit from this implantable optic.

Over the past 15 years, the VerisyseTM phakic IOL has established a reputation in Europe for stability, centration and visual acuity. When I saw how well European patients were doing with these anterior chamber implants, I decided to participate in the FDA trials of the VerisyseTM phakic IOL. This article recounts my experience with the VerisyseTM lens and how this technology will change the way we correct extreme myopia.
|
|
|
|
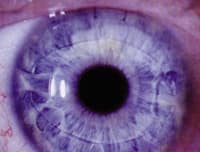
Verisyse™ phakic IOLs are held in place by anterior iris
stroma. |
New surgical perspective
The VerisyseTM phakic IOL is designed to correct myopia in patients whose extreme refractive error makes them poor candidates for LASIK correction. Verisyse™ implants are available in two sizes: A 6-mm optic that corrects between -5.00D and -15.00D of refractive error, and a 5-mm optic, which can correct up to 20.00D of refractive error.
Surgical technique is an important aspect of adopting VerisyseTM technology. Traditionally, we try to avoid touching the iris during eye surgery. However, to become successful VerisyseTM surgeons, we must overcome this mental barrier.
To implant the VerisyseTM phakic IOL you must become comfortable with manipulating the pa-tient's iris. The VerisyseTM lens is held in place over the pupil by threading anterior iris stroma through haptics at both ends of the lens. This system lets you center the optic over the pupillary entrance without worrying about its relationship to the corneoscleral limbus or the anterior chamber angle. If you're unhappy with your initial placement, you simply release the iris tissue from the haptics and move the optic to a more satisfactory position.
Positive results
More than 30 surgeons at 22 clinical sites have participated in FDA phase III clinical trials of the VerisyseTM phakic IOL. (For information about the study population, see "FDA Patient Selection Criteria.") A total of 560 patients with a mean refractive error of 12.59D received 971 implants.
Interim results from the phase III trial can be divided into three areas: Visual acuity, refractive predictability and stability.
Visual acuity. At 6 months post-op, 87.4% and 60.3% of patients had uncorrected visual acuities (UCVAs) better than 20/40 and 20/25, respectively. Data for patients with pre-op potential for 20/20 vision are even better. In addition, 10% of patients gained at least two lines of best-corrected visual acuity (BCVA), whereas only 0.5% lost two lines of BCVA, a favorable ratio of 20 to 1.
Refractive predictability. Eighty-six percent of patients achieved visual outcomes within 1.00D of their targets. Considering that average starting refractive error was almost -13.00D, this means 86% of patients were within 7% of their target outcomes. I attribute this degree of precision to needing only a combination of the patient's manifest refraction and anterior chamber depth. Total axial eye length doesn't affect patients' visual outcomes with VerisyseTM as it does in cataract surgery.
In addition, corneal wound healing has little to no effect on visual outcome. Historically, cataract patients treated through large incisions often developed progressive astigmatism secondary to wound slippage. However, 3 years after implanting VerisyseTM through 6-mm incisions, we have yet to detect progressive astigmatic drift in any study patients.
Stability. VerisyseTM patients show remarkably stable refraction and endothelial cell loss. At 3 years post-op, patients showed no significant change in mean refractive error. More importantly, VerisyseTM patients lost only 1.7% of their endothelial cell density (with most cell loss occurring within the first several months), compared with untreated patients whose cell densities decreased by 1.0%. This finding suggests that VerisyseTM lenses are not associated with accelerated endothelial cell loss.
|
FDA Patient Selection Criteria |
|
|
|
Know your options
When a patient comes to you for correction of extreme refractive error, how can you decide whether he's a better candidate for LASIK or for VerisyseTM? I've found that less myopic patients with thick, steep corneas, small pupils and shallow anterior chambers will do better with LASIK. Patients with thinner, flatter corneas, deep anterior chambers and mid-size to large pupils will do well with VerisyseTM. I followed these criteria to treat seven patients with LASIK in one eye and VerisyseTM in the other.
Preoperatively, the LASIK eyes averaged about 7.00D of myopia and the VerisyseTM eyes averaged 10.50D. Although post-op visual acuity was similar in both groups of eyes (20/25, P > 0.05), six of seven patients preferred the vision in their VerisyseTM eye even if they'd favored their less myopic (LASIK-treated) eye before surgery. Results from this small sample suggest the VerisyseTM phakic IOL is effective for the treatment of extreme myopia.
Alternative to LASIK?
Once VerisyseTM receives FDA approval, can we expect phakic IOLs to surpass LASIK as the refractive surgery of choice? As illustrated above, not all pa-tients are good candidates for phakic IOLs, just as not all patients will benefit from LASIK.
Patients who will most appreciate the VerisyseTM lens are the moderate to high myopes who were always told they'd never be candidates for refractive surgery. Data from the FDA phase III trial have proven otherwise. I predict that your VerisyseTM patients will be among the most satisfied patients in your refractive surgery practice, not only because they never expected to benefit from surgery, but also because they'll see better than they've ever seen with eyeglasses and contact lenses. The more patients I treat with Verisyse™, the more enthusiastic I become about this technology.
Third in a series sponsored by