at press time
Study to Assess Role of Glaucoma Imaging
Cleveland Clinic Will Coordinate $6 Million NEI Project.
The Cleveland Clinic's Cole Eye Institute will organize and coordinate a $6 million federal project that will be used to study advanced imaging techniques being used to diagnose and monitor glaucoma. The grant was awarded by the National Eye Institute (NEI), part of the National Institutes of Health.
|
|
|
|
The Stratus OCT will be one of the instruments
studied. |
|
The project, called "Advanced Imaging for Glaucoma" (AIG), is designed to develop and demonstrate the clinical use of several advanced imaging technologies, including optical coherence tomography (OCT), scanning laser polarimetry (SLP) and scanning laser tomography (SLT). All measure different parts of the eye, but are complementary. Advocates of these technologies say they are highly accurate and provide results more easily reproduced than traditional visual field testing.
"These new digital imaging methods provide objective and reproducible measurements of eye structures damaged by glaucoma, including optic nerve head, retinal nerve fiber layer and other retinal layers," says David Huang, M.D., Ph.D., an ophthalmologist at the Cole Eye Institute, and inventor of OCT technology. "Theoretically, these new tools should provide more accurate information than traditional tests."
But Dr. Huang says a long-term, large-scale clinical trial is necessary to determine how best to use these technologies to replace or complement traditional tests to improve glaucoma management. The AIG study will provide this information, says Dr. Huang, who will serve as the study's principal investigator.
The study will enroll 800 people: 200 patients without glaucoma; 200 patients with glaucoma who have related vision loss; and 400 patients either suspected of having glaucoma but not yet definitively diagnosed, or who have been diagnosed but are not yet experiencing vision loss.
The AIG researchers are primarily interested in studying the early stages of open-angle glaucoma, when diagnosis by advanced imaging may offer the greatest patient benefit. The study will test whether imaging detects glaucoma and glaucoma progression earlier than visual field testing. Positive data would justify the use of these new technologies to improve glaucoma management.
In addition to the Cleveland Clinic Cole Eye Institute, the Bascom Palmer Eye Institute in Florida and the University of Pittsburgh Medical Center will also participate in the AIG project.
For more information on enrolling patients in the AIG study, call research coordinator Kim Schach at (216) 445-6497.
Cooper Forms a Surgical Business
Its New Unit Acquires the AlphaCor Artificial Cornea.
The Cooper Companies, Inc. has formed a new surgical products business and acquired its first two products, the AlphaCor artificial cornea and AlphaSphere, a soft orbital implant. Both products were purchased from Argus Biomedical Pty Ltd., a privately owned Australian company.
|
|
|
|
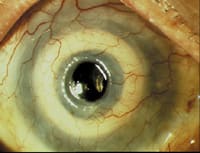
The AlphaCor artificial cornea post-op. |
Both the AlphaCor and AlphaSphere will be further developed and marketed to corneal surgeons by a newly formed business unit, CooperVision Surgical, Inc., a wholly owned subsidiary of CooperVision, Inc., the company's contact lens business.
The AlphaCor is suited for patients at high risk to reject a human cornea, or for patients for whom human transplants have failed. After AlphaCor is implanted, its dimensions, flexibility, water-absorbing properties and optics enable it to perform much like a human donor corneal graft.
The patented device is made in a single piece from a specialized polymer. It has a clear central optic surrounded by an opaque, sponge-like perimeter. Corneal cells grow into this spongy area and biointegration occurs between the device and the patient's cornea, anchoring the AlphaCor implant in place.
Argus, the developer of the AlphaCor, estimates that it could benefit about 20,000 patients each year.
More than 120 of the devices have been implanted worldwide. AlphaCor has received regulatory clearance in the United States and Canada, and is reimbursed by Medicare and by some private insurance plans.
AlphaSphere is currently under development. It's intended to occupy the space usually occupied by the eyeball after an eye has been lost to disease or trauma, and is designed to provide significant cosmetic benefits to the the patient.
"Starting with products like AlphaCor, we plan to acquire and develop other proprietary products for ophthalmic surgery that offer unique advantages over currently available therapies," said A. Thomas Bender, Cooper's chairman and CEO. "Ophthalmic surgeons are familiar with the CooperVision name, and we intend to leverage our strong brand image as we build a global franchise in this market."
Some Florida M.D.s Choose Self-Insurance
A New Support Group Offers Them Help.
With medical malpractice insurance rates continuing to skyrocket in South Florida, physicians in that part of the Sunshine State faced the prospect of either paying huge premiums or taking their chances and "going bare" on their own.
But now, a new service is helping about 100 Florida doctors through the process of self-insurance, offering them big discounts on legal fees and providing them with an array of resources that make practicing without insurance a more viable option.
The service, called Medical Defense Solutions (MDS), is the creation of Julie Danna, a partner in Delray Beach-based Gracey-Danna, Inc., whose basic business is arranging malpractice insurance coverage for Florida physicians.
Most of the physicians who join MDS are in specialties such as ob-gyn, where malpractice insurance premiums can run hundreds of thousands of dollars a year.
"We have a couple of ophthalmologists in MDS, but the rates haven't reached crisis levels for ophthalmology," notes Danna.
MDS offers physicians assistance with complying with state regulations for practicing without insurance. It also arranges hourly rate discounts of up to 50% with leading defense attorneys. Members involved in lawsuits can call on an experienced claims manager, and all members have access to the MDS lawsuit-prevention hotline, a quarterly newsletter, and regular risk management seminars.
In addition, MDS offers its members access to estate planners and tax experts who can advise them on implementing the best strategies for asset protection.
IN THE NEWS
Visudyne reimbursement. The Centers for Medicare and Medicaid Services (CMS) has raised the Medicare allowable reimbursement for Visudyne therapy for wet AMD from approximately $1,304 to $1,404. The CMS granted the exception to Visudyne after the drug's co-developers, QLT Inc. and Novartis Ophthalmics, appealed a provision in the Medicare Prescription Drug, Improvement and Modernization Act of 2003 that mandated a nearly 10% reduction in payments for drugs administered in a physician's office.
In a related development, effective April 1, ophthalmologists can now be reimbursed by CMS for using Visudyne therapy to treat AMD patients with occult and minimally classic lesions that are four disc areas or less in size and that show evidence of recent disease progression.
AMO acquisition. As we were about to go to press, Advanced Medical Optics said it had reached a definitive agreement to acquire Pfizer's surgical ophthalmology business for $450 million in cash.
Products included in the transaction are the Healon line of viscoelastics, the CeeOn and Tecnis IOLs used in cataract surgery, and the Baerveldt glaucoma shunt. These products together accounted for about $150 million in sales in 2003. We'll have moreon this in our June issue.
Macugen NDA. Eyetech Pharmaceuticals said it plans to file a New Drug Application (NDA) with the FDA for its wet AMD drug Macugen in the third quarter of this year. The company said its NDA will seek marketing approval for a 0.3-mg intravitreal dose of Macugen for the treatment of all three subtypes of wet AMD.
REFRACTIVE SURGERY UPDATE
CK approved for near vision. The FDA has approved Refractec's ViewPoint Conductive Keratoplasty (CK) System for performing the company's NearVision procedure. This procedure is the first FDA-approved vision technology that improves near vision in individuals above the age of 40 with presbyopia.
In a minimally invasive procedure that takes less than 3 minutes, NearVision CK uses radiofrequency energy to reshape the cornea and bring near vision into focus. The procedure is typically performed on just one eye, improving near vision without compromising the patient's binocular distance vision.
"The frustration many people feel with the on-again, off-again annoyance of reading glasses cannot be overemphasized," said Daniel S. Durrie, M.D., associate clinical professor, University of Kansas, and medical monitor for a CK clinical trial for treatment of presbyopia. "NearVision CK is just what baby boomers have been waiting for to help them get rid of their reading glasses and safely see like they did when they were young."
In a NearVision clinical trial, at 12-month follow-up, 98% of patients could see J5 (newspaper-size print) in the eye that was treated, while 87% could see 20/20 at distance and also read J3 (phonebook-size print). There were no reported serious, sight-threatening or unanticipated safety events.
CK was originally approved in 2002 for the temporary improvement of moderate, age-related hyperopia.
The New York Times ran a major feature article focusing on the CK procedure and its acceptance by baby boomers in its Sunday, April 11 issue.
Court finds for Nidek. A U.S. Court of Appeals for the Federal Circuit has upheld a lower court decision that Nidek's EC-5000 laser vision correction system didn't infringe on two technology patents owned by Summit Technologies, which is now a part of Alcon Laboratories. The court said that the plaintiff failed to present sufficient evidence to substantiate its infringement claim.
Alcon said it's disappointed with the appellate court's conclusion, but won't appeal the decision because it doesn't believe the cost of further appeals is warranted.
"Due to advances in technology since the lawsuit began, Alcon believes the outcome of this litigation will have little effect on its position in the refractive surgery market or on its financial results," an Alcon spokesperson stated.
LASIK for glaucoma patients. While glaucoma is a relative contraindication for refractive surgery, it's not an absolute one. So says Thomas W. Samuelson, M.D., writing in the April issue of Current Opinion in Ophthalmology.
Dr. Samuelson, clinical associate professor of ophthalmology at the University of Minnesota and a partner in Minnesota Eye Consultants, says the safety of LASIK in glaucoma patients remains unproven, but based on published literature, the procedure may be a viable option for at least some glaucoma patients.
For glaucoma patients considering LASIK, careful patient education and lifelong follow-up is a must, writes Dr. Samuelson. He also notes that clinicians must be aware that LASIK can have effects on the diagnostic testing of glaucoma patients. One example: Many investigators have found IOP to be reduced in individuals who've undergone LASIK, most likely due to reduced central corneal thickness.