Preservatives and Toxicity: Implications For
Therapy
Studies show short-term preservative
exposure is non-threatening to the epithelium.
BY ROBERT W. SNYDER, M.D., Ph.D.
The first two FDA-approved fourth generation fluoroquinolones are distinctly different. Gatifloxacin 0.3% (Zymar) is preserved with 0.005% BAK (benzalkonium chloride), and moxifloxacin (Vigamox) contains no preservative. The drugs used in cataract surgery are usually either a combination of gatifloxacin and Pred Forte -- both are preserved -- or a combination of unpreserved moxifloxacin and Econopred, which is preserved. With either combination, the theoretical advantage of being preservative-free is lost as the amount of BAK in Econopred surpasses the amount of BAK in either gatifloxacin or moxifloxacin.
Yeast, Mold and Bacteria
Yeast is a common source of ocular infections. The carriage rate of Candida spp in healthy individuals has been estimated as high as 80%.1 It's not surprising that Candida spp are common causes of mycotic exogenous endophthalmitis as most people are carriers.2 Candida parapsilosis has been implicated as the cause of epidemic exogenous endophthalmitis arising from the use of contaminated irrigating solution during surgery.3
With respect to fungi and bacteria as causes of ocular infection, Aspergillus spp of mold have been implicated in cases of endophthalmitis and fungal keratitis.2 Staphylococcus spp of bacteria are also found commonly in ocular infections. In the United States, coagulase-negative staphylococci are responsible for about 70% of postcataract surgery endophthalmitis, followed by Staphylococcus aureus, viridans group streptococci, as well as other Gram-positive and Gram-negative microorganisms.4-8

When looking at the safety and efficacy of fourth generation fluoroquinolones, we should take into account the U.S. Pharmacopoeia's (USP's) antimicrobial preservative efficacy criteria. Basically, the USP only requires stasis against Candida albicans and Asper-gillus niger. In contrast, the European Pharmacopoeia requires two logs of reduction at 7 days, and that's an important distinction. Of the fourth generation fluoroquinolones, only gatifloxacin with BAK meets the more stringent European standards.
The fluoroquinolones alone have no activity against fungi or Acanthamoeba, and I think that's the crux of the issue. Rupp and colleagues9 performed several studies of the fourth generation fluoroquinolones and their efficacy against various forms of yeast, mold and bacteria. They found that gatifloxacin preserved with 0.005% BAK effectively kills various potentially pathogenic yeasts, molds and bacterial strains of Staphylo- coccus to a greater extent than does unpreserved moxifloxacin. They also found that the anti-mold benefit at room temperature has the potential for preventing ocular infection caused by fungal contami- nation of ophthalmic solution bottles. These findings in yeast, mold and bacteria may have important implications in surgical and treatment settings.
In the yeast study, Rupp and colleagues looked at 20 strains of yeast that were inoculated with 105 and 106 colony-forming units (CFUs), incubated them at room temperature for 28 days, and then determined the postinoculation CFUs. At 4 hours, moxifloxacin consistently allowed more fungal recovery for all 20 strains than did gatifloxacin preserved with 0.005% BAK. Probably more importantly, at 7 days, moxifloxacin still had fungal recovery in 15 of the 20 strains, with both agents reducing the fungal recovery below the limit of detection in the other five strains. Rupp and colleagues concluded that the BAK effectively killed Candida and could reduce the risk of introducing yeast in the eyes and the surrounding structures during surgery.
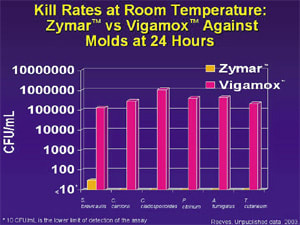
In the mold study, moxifloxacin again allowed more fungal recovery than did gatifloxacin preserved with 0.005% BAK for 12 fungal strains at all time points through day seven.10 In all fungal strains, the gatiflox-acin solution produced reductions in growth at least two logs greater than those produced by moxifloxacin. Eight strains treated with moxifloxacin had levels of at least 105 CFUs at 24 hours, while eight strains treated with gatifloxacin preserved with 0.005% BAK were reduced to levels of no recovery.
|
|
|
|
|
|

|
|

|
Antimicrobial Preservative Efficacy
Rupp11 performed an antimicrobial preservative efficacy test on gatifloxacin 0.3% preserved with BAK and unpreserved moxifloxacin to compare the performance of each. He conducted this experiment at room temperature against a strain of A niger. After 7, 14 and 28 days, gatifloxacin 0.3% preserved with BAK produced reductions in growth approximately two logs (99%) greater than those of moxifloxacin. The lower limit of detection for A niger was reached only with gatifloxacin. Gatifloxacin killed all strains of filamentous fungi tested at room temperature to a greater extent than unpreserved moxifloxacin. This antifungal benefit is likely to prevent ocular infection caused by mold contamination of ophthalmic solution bottles.12
In a bacteria study, seven strains of S aureus and one strain each of Staphylococcus hominis and Staphylococcus epidermidis were tested. All testing was performed in a water bath at 37° C. At intervals of 15, 30 and 60 minutes, samples of test solution were removed, neutralized and assayed for viable cells. For all four strains, the concentrations of CFUs of S aureus were reduced faster when exposed to gatifloxacin than when exposed to moxifloxacin.13 This difference in kill rates occurred at all measured time points. Similar differences in the kill rates occurred when gatifloxacin and moxifloxacin were tested against strains of ocular S epidermidis, S hominis and S aureus. For the strains of S hominis and S epidermidis, the logarithmic reduction in the gatifloxacin-treated samples was greater than in the moxifloxacin-treated samples at every time point. At 6 hours, moxifloxacin allowed more recovery of S aureus and Escherichia coli than did gatifloxacin after the same time period. At 24 hours, moxifloxacin allowed more recovery of S aureus than did gatifloxacin after the same time period. Data during these time periods represent cells that are not in the log phase of growth during an infection, but that are either in the stationary phase or otherwise not dividing or synthesizing DNA. The recovery of both species at both time points for gatifloxacin was below the level of detection for the assay.
The findings of this bacteria study indicate that gatifloxacin kills staphylococci faster than moxifloxacin does. Gatifloxacin's ability to provide faster anti-infective protection at body temperature than moxifloxacin may have important implications in a surgical setting.
Overcoming In-Vitro Resistance
In-vitro resistance doesn't necessarily translate to in-vivo resistance. In at least two rabbit models of gatifloxacin-resistant S aureus, aggressive treatment with gatifloxacin was sufficient to eradicate the infection.14,15
Finally, in-vivo use of BAK-preserved gatifloxacin may be sufficient for overcoming in-vitro resistance to gatifloxacin.16
These fourth generation fluoroquinolones are effective, and gatifloxacin preserved with BAK is more efficacious than gatifloxacin alone -- probably because the BAK provides additional efficacy. Studies of 0.005% BAK used for short time periods, such as the 1 to 2 weeks that we typically use these fluoroquinolones, indicate that the preservative is safe with respect to epithelial healing.
Ocular Surface Toxicity
An ideal topical antibiotic is nontoxic and noninflammatory. Donnenfeld and colleagues designed a simple experiment to examine the effects of gatifloxacin with BAK and unpreserved moxifloxacin on the ocular surface. Thirty subjects with no ocular pathology received doses of commercially available moxifloxacin and gatifloxacin solutions in masked bottles. One drop from each bottle was randomly placed in either the right or left eye two times at 1-minute intervals. The subject rested quietly with closed eyes for 5 minutes. A masked observer who did not administer the drops measured pupil size, conjunctival erythema, vascularity and anterior chamber cell and flare. Subjects graded pain, light sensitivity and irritation in each eye on a scale of 1 to 10.
The ocular tolerability study resulted in the following observations: Statistically significant conjunctival erythema was associated with moxifloxacin; no difference in the conjunctival erythema or vascularity was associated with gatifloxacin. Ocular irritation and pain were significantly less with gatifloxacin; while moxifloxacin produced a statistically significant reduction in pupil size.
Donnenfeld and colleagues concluded that moxifloxacin produces significant signs of inflammation, and gatifloxacin produces fewer clinical signs of inflammation on the ocular surface. They also theorized that the reduction in pupil size with unpreserved moxifloxacin was due to prostaglandin release.
Dr. Snyder is a professor and the head of the Department of Ophthalmology at the University of Arizona College of Medicine.
|
REFERENCES |
1. Murray PR, et al. Manual of Clinical Microbiology, 7th Ed, 1999; Weems JJ. Clin Infect Dis. 1992; Stern WH, et al. Ophthalmology, 1985. 2. Weems JJ Jr. Candida parapsilosis: epidemiology, pathogenicity, clinical manifestations, and antimicrobial susceptibility. Clin Infect Dis. 1992;14:756-766. 3. Stern WH, Tamura E, Jacobs RA, et al. Epidemic postsurgical Candida parapsilosis endophthalmitis. Clinical findings and management of 15 consecutive cases. Ophthalmology. 1985;92:1701-1709. 4. Karp CL, et al. Infectious keratitis after LASIK. Ophthalmology. 2003;110: 503-510. 5. Riddell IJ, McNeil SA, Johnson TM, et al. Endogenous Aspergillus endophthalmitis: report of 3 cases and review of the literature. Medicine (Baltimore). 2002;81:311-320. 6. Sridhar MS, Gopinathan U, Rao GN. Fungal keratitis associated with vernal keratoconjunctivitis. Cornea. 2003;22:80-81. 7. Callegan MC, Jensen H. Antibacterial activity of the 4th-generation fluoroquinolones gatifloxacin and moxifloxacin against ocular pathogens. Adv. Ther. In press. 8. Callegan MC, Engelbert M, Parke DW 2nd, et al. Bacterial endophthalmitis: epidemiology, therapeutics, and bacterium-host interactions. Clin. Microbiol. Rev. 2002;15:111-124. 9. Rupp D, et al. The antimicrobial preservative efficacy of Zymar and Vigamox against yeast isolates. Abstract, OMIG. 2003. 10. Reeves. Unpublished data. 2003 11. Rupp. Unpublished data. 2003. 12. Allergan data on file. 13. Allergan data on file. 14. Mah FS, Romanowski EG, Yates KA, et al. The successful treatment of gatifloxacin-resistant Staphylococcus aureus keratitis with gatifloxacin (Zymar) in a NZW rabbit model. OMIG. 2003. 15. Tungsiripat T, Sarayba MA, Kaufman MB, et al. Fluoroquinolone therapy in multiple drug-resistant staphylococcal keratitis after lamellar keratectomy in rabbit model. Am J Ophthalmol. 2003;136:76-81. 16. Allergan data on file. |