Meeting the Challenge of
Rising Resistance
Fourth generation fluoroquinolones may
overcome and delay resistance.
BY FRANCIS S. MAH, M.D.
Emerging ocular resistance poses a significant challenge to clinicians. And identifying the anti-infective best-equipped to handle that challenge gives us an opportunity to rethink and refine clinical protocols.
Postoperative infection rates appear to be rising. Recent data suggest that endophthalmitis rates, usually 1 in 1,000, can be as high as 1 in 4001; and areas in Mexico have reported the rate as 1%, or 1 in 100.
At the Campbell Laboratory, we found that about 94% of endophthalmitis is caused by Gram-positives, and the remaining 6% by Gram-negatives, mirroring the findings of the 1990 Endophthalmitis Vitrectomy Study.2
Antibiotic resistance is one of the primary causes of the increasing endophthalmitis rate specifically, and the increasing rate of postsurgical infections, in general. Among other things, surgical complications and choice of prophylactic antimicrobials also have a role.
Wound Architecture
In terms of cataract surgery, it's becoming increasingly clear that wound architecture is related to rising endophthalmitis rates, as well.3 Clear corneal incisions have become the popular choice because they enable sutureless surgery and rapid visual rehabilitation after phacoemulsification. The problem is that a relationship may exist between clear corneal incisions and a higher risk of postoperative infection. And the risk may in-crease with transient reductions in IOP.3
Several studies have shown a greater incidence of endophthalmitis with clear corneal incisions than with scleral tunnel incisions. Consider the following:
► A study in Canada4 showed a 2.6- to 3.5-fold increased risk of endophthalmitis with clear corneal incisions versus superior scleral tunnels.
► A study in Japan5 showed a 4.6-fold increased risk of endophthalmitis with temporal corneal incisions versus superior sclerocorneal incisions.
► Buzard and colleagues reported a 15-fold increased risk with clear corneal versus scleral tunnel incisions.6
► A St. Louis group reported a 3.36-fold increased risk of endophthalmitis with corneal incisions versus scleral tunnels.7
This appears to be a trend, averaging a 2- to 4-fold increased risk of endophthalmitis with clear corneal incisions compared to scleral tunnel incisions.
A study on the dynamic morphology of clear cor-neal cataract incisions suggests these incisions may result in poor wound apposition and increased potential for fluid flow across the cornea and into the anterior chamber.8 This can be compared to a sucking action, drawing the periocular fluid into the eye and, therefore, increasing the attendant risk for endophthalmitis. It is critical, therefore, to have an efficacious anti-infective on board to combat the potential bacteria that enter the eye.
Increasing Resistance
Two published reports document increasing rates of resistance to fluoroquinolones. Goldstein9 from our lab reported an increase in laboratory resistance of Staphylococcus aureus to fluoroquinolones from 5.8% in 1993 to 35% in 1997. And from Bascom Palmer, Alexandrakis and colleagues10 reported an increase in laboratory resistance of S aureus to fluoroquinolones from 11% in 1990 to 28% in 1998.
In the Campbell Lab, S aureus has become in-creasingly resistant to the earlier generations of fluoroquinolones. In 1993, just 2 years after the release of ciprofloxacin (Ciloxan) and ofloxacin (Ocuflox), susceptibility was 100%.11 From 1993 to 1997, we've shown a sevenfold increase in resistance to S aureus.
What can we do about resistant pathogens? Some strategies to consider include:
► Seek new targets for antibiotics.
► Control the use of antibiotics.
► Diagnose rapidly so that we're acting against the offending pathogen.
► Employ good hygiene and sanitation practices, which have been shown to decrease epidemics as well as infections.
► Use surveillance to follow the rates of resistance and to document the pathogens causing infections.
► Develop vaccines to prevent infections.
Developing novel antibiotics, such as the fourth generation fluoroquinolones, is another option, and it's ultimately the one that has been embraced by the research and science communities.
8-Methoxy Group
The improved in vitro activity of the fourth generation fluoroquinolones against Gram-positives and resistant bacteria is due to one substitution on the molecule -- the 8-methoxy group, or CH3.12 This methoxy group has advanced the technology of fluoroquinolones and increased activity against not only the Gram-positives but also against resistant bacteria. Of the 10,000 fluoroquinolones that have been reported, only gatifloxacin (Zymar) and moxifloxacin (Vigamox) have this 8-methoxy group.13
The primary targets for the previous generation of fluoroquinolones were DNA-gyrase in the Gram-negatives and topoisomerase-4 in Gram-positives, but those older fluoroquinolones didn't bind well to topo-isomerase-4. However, the fourth generation fluoroquinolones bind with a strong affinity to both DNA-gyrase and topoisomerase-4. Because of this, the fourth generation fluoroquinolones now cover the Gram-positives, including Streptococcus pneumoniae, as well as resistant bacteria.

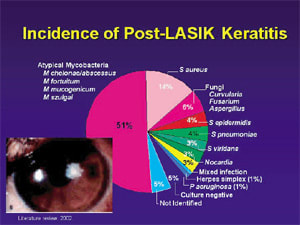
Mycobacteria and Penetration
Because mycobacteria are becoming increasingly problematic in ophthalmic surgery, the New York Eye and Ear Infirmary did a study looking at mycobacteria that cause endophthalmitis and LASIK infections. This study showed that in the M fortuitum group, the fourth generation fluoroquinolones had the lowest MICs, and that in the M chelonae group, gatifloxacin had the lowest MICs.14
The fluoroquinolones were the first class of antibiotics that could penetrate the cornea and the anterior chamber to a significant degree. Ofloxacin was the gold standard in terms of penetration; it penetrated much better than ciprofloxacin. Gatifloxacin and ofloxacin seem to have similar penetration rates.15

A patient presented with a recurrent erosion and a resultant corneal ulcer. I prescribed a fourth generation fluoroquinolone and cefazolin, which is our new protocol for treating serious or non-responding bacterial keratitis. Two days later, the laboratory reported methicillin-resistant S aureus (MSRA) with resistance to the fourth generation fluoroquinolones. The report indicated that only bacitracin or vancomycin would work. However, the patient was feeling and looking better. I stopped the cefazolin, but kept her on the fourth generation fluoroquinolone. Ten days later, she completely resolved. It's great to see that these drugs work in the lab, but seeing how they work in the clinical setting is what's ultimately important.
It's become increasingly common for antibiotic susceptibility testing to report resistance, despite the fact that the patient is clinically responding to therapy. We decided to look at this scenario in rabbit models. We injected S aureus into the corneal stroma and treated with gatifloxacin 0.3%, levofloxacin 0.5% (Quixin) and ciprofloxacin 0.3%. We examined the eyes in a masked fashion and found that the gatifloxacin eyes looked much better than the levoflox-acin eyes, despite the fact that levofloxacin has a lower MIC. The gatifloxacin was effective in reducing the bacterial colony counts, and the gatifloxacin eyes were the most improved, with the least toxicity.
So, in our study, which we presented at the 2004 Ocular Microbiology and Immunology Group (OMIG) meeting, gatifloxacin 0.3% was very effective in reducing the counts of these MRSA strains.
Improved Coverage
The earlier generation of fluoroquinolones had their time, but they're no longer useful and potentially, continued use could actually decrease the usefulness by increasing resistance. We need topical antibiotics that cover Gram-positives and Gram-negatives, as well as atypicals. They have to have a high level of antimicrobial activity and kill rapidly, like the fluoroquinolones do.
Gatifloxacin 0.3% offers improved activity against Gram-positives, improved activity against atypicals and retained activity against Gram-negatives. The Gram-positive pathogens, which were resistant to the previous generations of fluoroquinolones, are now susceptible. Gatifloxacin -- at least in the rabbit model -- is less toxic than levofloxacin and ciprofloxacin, and it has the ability to overcome and delay the development of resistance.
Dr. Mah is an assistant professor of ophthalmology at the University of Pittsburgh, and he is co-medical director of the Charles T. Campbell Ophthalmic Microbiology Laboratory.
|
REFERENCES |
1. Jensen MK, Fiscella RG. Comparison of endophthalmitis rates over four years associated with topical ofloxacin vs. ciprofloxacin. ARVO, 2002; Ft. Lauderdale, Fla. 2. Speaker MG, Milch FA, Shah MK, et al. Role of external bacterial flora in the pathogenesis of acute postoperative endophthalmitis. Ophthalmology. 1991;98:639-649. 3. Taban M, Sarayba MA, Rao B, et al. Dynamic morphology of clear corneal cataract incision [abstract]. Invest Ophthalmol Vis Sci. 2003;2578. 4. Colleaux KM, Hamilton WK. Effect of prophylactic antibiotics and incision type on the incidence of endophthalmitis after cataract surgery. Can J Ophthalmol. 2000;35:373-378. 5. Nagaki Y, Hayasaka S, Kadoi C, et al. Bacterial endophthalmitis after small incision cataract surgery: effect of incision placement and intraocular lens type. J Cataract Refract Surg. 2003;29:20-26. 6. Buzard K, et al. Endophthalmitis: Scleral tunnel v. clear corneal incision. In: Blue Line Incision and Refractive Phacoemulsification. Slack Inc: 2001. 7. Cooper BA, et al. Case-control study of endophthalmitis after cataract surgery comparing scleral tunnel and clear corneal wounds. Am J Ophthalmol. 2003;136:300-305. 8. McDonnell PJ, Taban M, Sarayba M, et al. Dynamic morphology of clear corneal cataract incisions. Ophthalmology. 2003;110:2342-2349. 9. Goldstein et. al. Emerging fluoroquinolone resistance in bacterial keratitis: A 5-year Review. Ophthalmology. 1999;106:1313-1318. 10. Alexandrakis et. al. Shifting trends in bacterial keratitis in South Florida and emerging resistance to fluoroquinolones. Ophthalmology. 2000;107:1497-1502. 11. Kowalski RP, et al. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin, and ofloxacin using bacterial keratitis isolates.Am J Ophthalmol. 2003;136(3):500-505. 12. Fukuda H, Kishii R, Takei M, Hosaka M. Contributions of the 8-methoxy group of gatifloxacin to resistance selectivity, target preference, and antibacterial activity against Streptococcus pneumoniae. Antimicrob Agents Chemother. 2001;45:1649-1653. 13. Ince D, Hooper DC. Mechanisms and frequency of resistance to gatifloxacin in comparison to AM-1121 and ciprofloxacin in Staphylococcus aureus. Antimicrob Agents Chemother. 2001;45:2755-2764. 14. Shah MK, et al. Will the topical fourth generation fluoroquinolones become the antibiotics of choice for treating atypical mycobacteria-related eye disease? Abstract; 2003, ARVO. 15. Levine. J Cataract Refract Surg. In press. |








