GLAUCOMA MANAGEMENT PERSPECTIVES
- Second in a Series of Four
Arresting Glaucoma's ADVANCE
Careful optic nerve evaluation can detect the disease in time
to halt progression through timely treatment.
Patients can go on enjoying a quality of life unimpaired by visual loss.
By Steven T. Simmons, M.D.
Take this FREE CME course online at www.visioncarecme.com.
It's been said that the goal of glaucoma treatment is to prevent patients from going blind in their lifetime. No doubt, many glaucoma patients consider this objective unacceptable. Rather than preventing blindness, patients and clinicians feel the goal should be to preserve the present visual function and prevent further disease progression.
From the patient's perspective, any progressive loss indicates that his or her glaucoma is not under control -- that his vision will continue to worsen and his ability to perform important daily activities will be severely impaired or lost. Studies have shown that glaucomatous vision loss can have a significant effect on a patient's overall quality of life.1-4 Even a small visual field defect, depending on its location, can make certain activities such as driving or negotiating stairs very difficult. Additional evidence indicates that once glaucoma reaches an advanced stage, it's more difficult to prevent further progression.5,6
Considering how strongly quality of vision can affect quality of life, the appropriate goal of glaucoma treatment is to prevent disease progression. This article describes the relationship between optic nerve changes and glaucomatous visual field loss. This causal link underscores the importance of optic nerve examinations in patient evaluations. The information gained from optic nerve evaluations serves as a guide to treatment decisions.
|
|
|
|
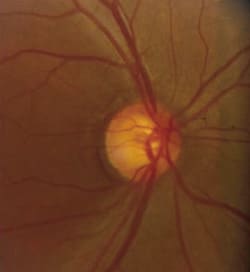
Fig. 1. Preperimetric glaucoma typically manifests as early
glaucomatous cupping of the optic nerve, thinning of the neuroretinal rim and baring of the circumlinear vessel. |
|
Structural damage and vision loss
The defining manifestation of glaucoma is the death of retinal ganglion cells (RGCs). This results in optic nerve head cupping, retinal nerve fiber layer (NFL) loss and visual field defects. Recognizing these changes early on is critical to obtaining successful outcomes. The retina has enough neural reserve that many RGCs can be lost before visual function is affected. Evidence from human eyes suggests that as many as 25% to 35% of RGCs must be lost from a given part of the retina before a visual field defect for that area is detectable.7 Experimental glaucoma models in primates suggest that, in some parts of the retina, RGC loss could be as high as 50% before visual function is impaired.8
From a clinical perspective, this means that by the time glaucomatous visual field defects are evident, neural damage is advanced, the neural reserve has been depleted and any further neural damage will likely result in progressively worsening visual field loss. To preserve as much vision as possible -- or preferably prevent vision loss from occurring at all -- glaucomatous optic nerve damage must be detected as early as possible and then halted.
Several clinical studies have demonstrated that measurable structural damage often precedes measurable visual field loss.9-12 In the Ocular Hypertension Treatment Study (OHTS), roughly 55% (69/125) of patients who progressed to glaucoma developed optic disc abnormalities with no concurrent visual field defects.12 In 1977, Sommer et al. demonstrated that 14 patients with glaucomatous visual field loss had clear NFL abnormalities on average 18 months before visual field defects were detected. In a later study, Sommer and colleagues noted that six of 10 patients who developed Goldmann visual field loss had abnormal optic discs 6 years before the field loss.10 A study by Airaksinen et al. found that eight of 25 patients with disc hemorrhage and no field loss subsequently developed field loss over a mean of 6 years; the NFL in these patients became abnormal in the area of the hemorrhage and preceded field loss by 1 to 2 years.11
These studies indicate that structural damage in glaucoma can be observed several years before a functional loss develops. In the OHTS study, approximately 35% (44/125) of patients who progressed to glaucoma developed reproducible visual field changes before optic disc deterioration was detectable. This underscores the urgency of recognizing early signs of optic nerve damage.
Although structural damage precedes functional loss, we're limited in our ability to measure that damage. Researchers estimate that 30% to 40% of RGCs must be lost before the optic disc or NFL can be clearly identified as abnormal. This is virtually identical to the estimated RGC loss that must occur before visual field defects can be detected. This explains why optic disc abnormalities may not be identified in some patients until visual field loss is imminent or has already occurred.
Considering the evidence, observable optic nerve damage indicates that a patient left untreated is at high risk of visual field loss within a fairly short time.
|
|
|
|
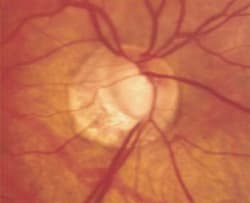
Fig. 2. Advanced glaucomatous cupping of the optic nerve with notching and loss of the
neuroretinal rim and extensive peripapillary atrophy. A corresponding superior arcuate field defect, threatening fixation, was seen on
standard automated perimetry. |
Early detection is critical
The large amount of RGC loss underlying detectable visual field defects in glaucoma means that our definition of early glaucoma should not be based solely on early visual field loss or high IOP. Only patients with preperimetric glaucoma -- identifiable optic disc abnormalities but no field loss -- should be considered to have early or mild glaucoma (See Figure 1). Once a visual field defect is detectable, the patient can be said to have moderate to severe glaucoma, depending on the degree of field loss (See Figure 2).
Glaucoma testing is appropriate for any patient with visual complaints characteristic of glaucoma or identifiable risk factors for glaucoma. Risk factors include:
- Family history of glaucoma
- Elevated IOP
- Diabetes
- Age over 50 years
- African-American heritage.
Medicare has recognized the importance of early glaucoma detection by approving reimbursement for glaucoma screening tests for high-risk individuals.13
Although structural damage precedes functional vision loss in glaucoma, and optic nerve abnormalities usually can be detected before visual field loss occurs, this isn't always the case. Thus, the initial glaucoma exam should include both an optic nerve evaluation and visual field testing.
|
|
|
|
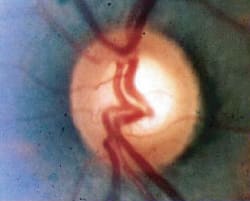
Fig. 3. Advanced loss of the neuroretinal rim and diffuse thinning of the nerve fiber layer in a patient with advanced
glaucoma. |
|
Optic nerve evaluation
Early changes associated with glaucomatous optic neuropathy include: Generalized enlargement of the cup, focal enlargement of the cup (Figure 3), increased cup/disc ratio, acquired pit of the optic nerve, notching or narrowing of the neuroretinal rim, isolated disc hemorrhage, peripapillary atrophy (Figure 4), focal loss of the nerve fiber layer (Figure 5), translucency of the neuroretinal rim, baring of circumlinear vessel (Figure 6) and asymmetric cupping between the patient's two eyes.14
You can observe pathological changes in the optic nerve using a direct ophthalmoscope or a slit-lamp biomicroscope with a posterior pole lens. Stereophoto graphy of the optic disc, retinal NFL images and topographical assessment of the optic nerve head are useful in evaluating optic nerve health as well. Document the state of the optic nerve or NFL with a photograph or digital image; without these, you can't detect further structural deterioration in preperimetric glaucoma and glaucoma suspects. A detailed drawing of the optic disc is not as good as photography or digital imaging, but it's sufficient if no other method is available.
|
|
|
|
Fig. 4. Peripapillary atrophy in a patient with early cupping and early loss of the inferior neural rim. Beta-zone peripapillary |
Stereophotography of the optic disc is the most widely used and well-studied method for evaluating the optic nerve. Also valuable are digital imaging techniques such as scanning laser polarimetry (SLP) with a GDx machine, Heidelberg Retina Tomography (HRT) and optical coherence tomography (OCT), especially with the Stratus version. The literature offers insufficient evidence to recommend any of these techniques over any other. Therefore, you should base your choice of technique on practical considerations such as the availability of the equipment or the expertise to conduct the examination and interpret the results accurately. (See, "Methods of Optic Nerve Evaluation.")
Medicare in some areas will reimburse for fundus photography and digital imaging of the optic nerve in glaucoma patients. Reimbursement for fundus photography will be approved for new or established glaucoma patients but can't be billed on the same day as a visual field test or digital imaging.
Visual field evaluation
Standard automated static threshold perimetry (SAP) is the preferred technique for evaluating the visual field in glaucoma, according to the American Academy of Ophthalmology Preferred Practice Patterns. Other visual field tests -- including short wavelength automated perimetry (SWAP) and frequency doubling technology (FDT) -- are also available and increasingly popular for evaluating glaucoma suspects and patients with early disease; these methods can document deficits earlier than SAP.15
The typical visual field defects in primary open-angle glaucoma include enlargement of the blind spot, nasal step, paracentral scotoma, generalized depression and arcuate defects.14
|
|
|
|
Fig. 5. Focal loss of the retinal nerve fiber layer associated with early atrophy of the inferior neuroretinal rim in a patient with primary open angle glaucoma
(POAG). |
|
The impact of field loss
The impact of glaucomatous visual field loss on patients' quality of life has long been underappreciated. Quality-of-life analyses have only recently been added to NEI-sponsored glaucoma clinical trials and a glaucoma-specific quality-of-life instrument is still under development.1,2,16 These surveys show a clear impact of the quality of vision on the quality of life. The magnitude of that impact correlates directly to the degree of visual field loss.
Generally, visual field loss in glaucoma first manifests as a loss of peripheral vision, while central vision may not be affected until the disease is far-advanced.17 Even patients who retain excellent central vision, however, may experience significant visual disability and difficulty performing daily activities.
The activities most likely to be affected are those associated with glare and lighting and tasks involving peripheral vision.1 This leads to problems such as tripping and bumping into objects, difficulty with stairs and trouble finding dropped objects.1,18 Patients with glaucoma have difficulty reading and watching television and a higher risk of falling.2,4,19 Glaucoma has been identified as a significant risk factor in automobile accidents among the elderly.3 These functional impairments have a strong impact on vision-related quality of life.20,21,22 They may have a societal impact beyond the afflicted individual.
The degree of visual disability and its impact on the quality of life correlates with the degree of visual field loss.20,21,22 Clearly, every effort must be made to prevent disease progression and preserve as much visual function as possible in glaucoma patients.
Failure to prevent disease progression in early glaucoma can make it more difficult to prevent further progressive visual field loss. Studies show that IOP must be reduced to very low levels (< 15 mm Hg or 40% from baseline) in patients with advanced disease to slow or halt further vision loss.5,6,14 Even with today's potent IOP-lowering medications, such low pressures are difficult to achieve and maintain.
The annual direct costs of treatment are greater in patients with more advanced disease.23 These encompass office visits, surgery, medications, field testing, low-vision services and other specialized services.
Identifying progression
Identifying progression in glaucoma requires a thorough initial examination with meticulous documentation of the optic nerve and visual fields. In glaucoma suspects and patients with early glaucoma, optic nerve deterioration may be the first sign of disease progression; this cannot be detected without good baseline documentation (see Figure 7). Clinicians must look for signs of disease progression such as further cupping, notching or narrowing of the neuroretinal rim, disc hemorrhage peripapillary atrophy, focal loss of the optic nerve layer, translucency of the neuroretinal rim and baring of the circumlinear vessel. Regular follow-up evaluation of both the optic nerve health and visual fields is required to detect progression at all stages of glaucoma. Optic nerve evaluations are particularly important in early disease. In more advanced disease, visual field testing coupled with optic nerve evaluations can help in detecting progression.
|
|
|
|
Fig. 6. Profound baring of the inferior circumlinear vessel in a patient with pseudoexfoliative glaucoma. |
Reducing progression risk
The AAO Preferred Practice Patterns for primary open-angle glaucoma specifically states that treatment efficacy should be defined in terms of how well disease progression is slowed or halted.14 These guidelines describe how the first step for each patient is to estimate the IOP below which further optic nerve damage is unlikely to occur. The goal of therapy is to maintain IOP below this level. If the initial target IOP fails to curb progression, the target should be further lowered.
Numerous clinical trials show that IOP levels below 18 mm Hg are necessary to slow or halt progressive visual field loss in glaucoma. In the Advanced Glaucoma Intervention Study (AGIS), patients with IOP consistently below 18 mm Hg over a 6-year follow-up period had mean progression close to zero. In a study by the Collaborative Normal Tension Glaucoma Group (CNTG), eyes that achieved and maintained a 30% reduction in IOP were significantly less likely than untreated eyes to show progression of optic disc cupping and/or visual field defects.24 A study by Mao and associates found that all eyes with an average IOP less than 17 mm Hg during 4 to 11 years of follow-up remained stable, while all eyes with an average IOP over 21 mm Hg had progressive optic disc cupping and/or functional vision loss.25
Studies suggest that IOPs of 15 mm Hg or lower may be required to prevent further progression in patients with advanced visual field loss.5,6 The Early Manifest Glaucoma Trial (EMGT) showed that each 1 mm Hg of IOP lowering results in approximately a 10% decrease in the risk of disease progression.26
Most importantly, the results of the Collaborative Initial Glaucoma Treatment Study (CIGTS) demonstrated that low rates of progression occurred when IOP was lowered to a predetermined, individualized target.28 During the 5-year follow-up period, clinically significant visual field loss occurred in fewer than 11% of patients treated with IOP-lowering medications; such field loss developed in less than 14% of patients who underwent surgery to achieve an individualized target.29 In contrast, the rate of disease progression among treated patients in the EMGT trial -- in which all patients received the same therapy regardless of individual need -- was 30% after 4 years and 45% over the duration of the study.26
These study results highlight the importance of setting a target IOP as a goal of therapy, and indicate that initial target pressures should be below 18 mm Hg for most patients (below 15 mm Hg for those with more advanced disease). Unfortunately, a recent survey revealed that common clinical practice falls short of these goals.29 In a survey of eyecare records from 395 working-age glaucoma patients enrolled in managed care plans, fewer than 2% had a target level specified. Less than 48% of patients with mild glaucoma achieved IOPs below 19 mm Hg, and approximately 35% of patients with moderate to severe glaucoma achieved IOPs below 16 mm Hg. If these practice trends continue, the rate of progressive glaucomatous visual field loss is likely to remain high.
Curtailing glaucoma's impact
Our growing understanding of the impact of glaucomatous visual field loss makes it clear that preventing disease progression should be the overriding goal of glaucoma therapy. This can best be achieved by:
- Making every effort to detect glaucoma early
- Setting IOP-lowering goals that are likely to prevent progression
- Monitoring patients regularly for signs of optic nerve or visual field deterioration.
Because neural damage occurs before glaucomatous visual field loss, optic nerve evaluations are critical for detecting and monitoring glaucoma suspects and patients with early glaucoma. A great deal of neural damage occurs before optic nerve or visual field damage can be measured.
|
|
|
|
Fig. 7. Progressive loss of the inferior neuroretinal rim and baring of the circumlinear vessel in a patient with IOPs in the low 20s for more than 9
years. |
|
Treatment to lower IOP should begin as soon as structural or functional defects are clearly apparent. Waiting for the disease to progress before initiating treatment could rob the patient of important visual function and make the condition more difficult and costly to treat in the future. Once treatment begins, its efficacy is defined by how well it prevents disease progression rather than on the basis of IOP-lowering.
Many potent IOP-lowering medications and surgical procedures are available to treat glaucoma. We have the tools to limit or prevent glaucomatous vision loss for most patients. The medical community's effort to identify glaucoma patients early and treat them with effective therapies could greatly reduce the disease's devastating impact.
Steven T. Simmons, M.D., is co-director of the Glaucoma Consultants of the Capital Region in Albany, N.Y. He also serves as the medical advisor to the Northeastern New York Sight Conservation Society. In addition, he is an associate clinical professor of ophthalmology at Albany Medical College and senior editor of The Basic Science Series in glaucoma for the American Academy of Ophthalmology.
|
Methods of Optic Nerve Evaluation |
| Optic disc stereophotography. Stereoscopic photographs of the optic nerve create a 3-dimensional image that lets you analyze cup depth and excavation as well as rim contour. Stereophotography correlates with visual field defects and often can detect optic nerve abnormalities before field defects are seen. Stereophotography is fast and easy for the patient. However, it requires specialized equipment, a skilled photographer, an expert evaluation and considerable time. Scanning laser polarimetry of the retinal nerve fiber layer. Scanning laser polarimetry (SLP) measures the retinal nerve fiber layer by detecting the degree to which polarized light is retarded as it passes through the retina; the signal comes from light reflected off the retina. This test is performed with the GDx VCC (Laser Diagnostic Technologies). This machine generates a measurement in 0.8 seconds through an undilated pupil. A corneal compensator removes the portion of the signal attributed to the anterior segment. SLP is user-friendly, and the manufacturer continues to refine the GDx technology and software to make it more reproducible and applicable to clinical practice. Heidelberg Retina Tomography. Heidelberg Retina Tomography (HRT, Heidelberg Engineering) uses a confocal scanning laser ophthalmoscope to obtain 3-dimensional images of the optic disc and the peripapillary retina. This is a user- and patient-friendly technique that's fairly easy to learn. HRT has the longest track record of the automated imaging techniques and the most publications on its use. This technique seems to be as good as or better than disc photographs for detecting structural damage. New software has improved clinicians' ability to detect progressive focal topographical changes over time. Continued research is needed to better define the role of HRT in everyday clinical practice. Optical coherence tomography. Optical coherence tomography (Stratus OCT, Carl Zeiss Meditec) discriminates retinal layers based on differences in the time delay of light echoes from scanned retinal tissue. This method allows imaging of the peripapillary retina, optic nerve head and macular region. It can determine NFL thickness, macular thickness and cup/disc ratio. As with all imaging technologies, this technique can be difficult to apply in patients with substantial media opacities. RNFL data are compared with normative values to assist in glaucoma diagnosis. Continued research and software refinements are necessary before OCT is used routinely to identify progression in glaucoma patients. |
|
Visual Field Evaluation Methods |
Standard automated perimetry (SAP). Standard automated perimetry measures differential light sensitivity at multiple locations in the peripheral visual field. It's the oldest, best-documented and most widely available visual function test based on differential sensitivity to white light. SAP is suitable for detecting glaucoma over a range of severities and for monitoring disease progression. The test is suitable for most patients and doesn't require pupil dilation. The techniques for conducting the test and interpreting the results are standardized and easy to learn, and the results are intuitively comprehensible to physicians and patients. Frequent SAP testing can identify early as well as late disease progression. Short-wavelength automated perimetry (SWAP). Short-wavelength automated perimetry is similar to SAP, but it uses a blue stimulus on a yellow background specifically to test the functioning of a subset of RGCs (small bistratified ganglion cells). This technique can be applied to most of the same patients as SAP, but it's more sensitive to pre-retinal light absorption due to lens yellowing and other media opacities. SWAP is more sensitive than SAP in documenting early glaucomatous vision loss. It appears to be most useful in testing glaucoma suspects and patients with early glaucoma who still retain excellent vision. However, the test is long and onerous for both the patient and the staff. Frequency doubling technology (FDT) perimetry. In FDT perimetry, a low-spatial-frequency sinusoidal grating undergoing counterphase flicker serves as the target against a high-luminous background. This type of perimetry is relatively new and is still being evaluated. But it appears to have good sensitivity, specificity and reproducibility in detecting visual field loss due to glaucoma or other neurological disorders. FDT appears to distinguish mild, moderate and severe visual field loss very well. FDT results are relatively unaffected by moderate amounts of refractive error or by variations in pupil size. In early studies, FDT was at least as sensitive to early visual field loss as SWAP. FDT's ability to detect disease progression is still under investigation. Because FDT is quick and patient-friendly, it's useful for patient screening. FDT may prove invaluable in following glaucoma suspects and patients with early disease. |
Sponsored by

Supported by an unrestricted educational grant from

REFERENCES
1. Nelson P, Aspinall P, O'Brien C. Patients' perception of visual impairment in glaucoma: a pilot study. Br J Ophthalmol. 1999 May;83(5):546-52.
2. Nelson P, Aspinall P, Papasouliotis O, Worton B, O'Brien C. Quality of life in glaucoma and its relationship with visual function. J Glaucoma. 2003 Apr;12(2):139-50.
3. McGwin G Jr, Owsley C, Ball K. Identifying crash involvement among older drivers: agreement between self-report and state records. Accid Anal Prev. 1998 Nov;30(6):781-91.
4. Ramrattan RS, Wolfs RC, Panda-Jonas S, Jonas JB, Bakker D, Pols HA, Hofman A, de Jong PT. Prevalence and causes of visual field loss in the elderly and associations with impairment in daily functioning: the Rotterdam Study. Arch Ophthalmol. 2001 Dec;119(12):1788-94. Erratum in: Arch Ophthalmol 2002 Apr;120(4):525.
5. Grant WM, Burke J Jr. Why do some people go blind from glaucoma? Ophthalmology 1982;89(9):991-998.
6. Shirakashi M, Iwata K, Sawaguchi S, Abe H, Nanba K. Intraocular pressure-dependent progression of visual field loss in advanced primary open-angle glaucoma: a 15-year follow up. Ophthalmologica. 1993;207(1):1-5.
7. Kerrigan-Baumrind LA, Quigley HA, Pease ME, Kerrigan DF, Mitchell RS. Number of ganglion cells in glaucoma eyes compared with threshold visual field test in the same persons. Invest Ophthalmol Vis Sci. 2000;41:941-748.
8. Harwerth RS, Crawford ML, Frishman LJ, Viswanathan S, Smith EL 3rd, Carter-Dawson L. Visual field defects and neural losses from experimental glaucoma. Prog Retin Eye Res. 2002 Jan;21(1):91-125.
9. Sommer A, Miller NR, Pollack I, Maumenee AE, George T. The nerve fiber layer in the diagnosis of glaucoma. Arch Opththalmol. 1977;95:2149-56.
10. Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77-83.
11. Airaksinen PJ, Mustoned E, Alanko HI. Optic disc hemorrhages precede retinal nerve fibre layer defects in ocular hypertension. Acta Ophthalmol (Copenh) 1981; 59:627-41.
12. Kass MA, Heuer DK, Higginbotham EJ, et al. The Ocular Hypertension Treatment Study, Archives of Ophthalmology. 2002;120:701-713.
13. Talsma J. New glaucoma screening benefit targets high-risk, underserved population. Ophthalmology Times. March 15, 2002.
14. American Academy of Ophthalmology Preferred Practice Pattern. Primary Open Angle Glaucoma. 2000.
15. Delgado MF, Nguyen NTA, Cox TA et al. Automated perimetry: a report by the America Academy of Ophthalmology. Ophthalmology 2002;109:2362-2374.
16. Janz NK, Wren PA, Lichter PR et al. The Collaborative Initial Glaucoma Treatment Study: interim quality of life findings after initial medical or surgical treatment of glaucoma. Ophthalmology 2001;108(11):1954-1965.
17. Coleman AL. Glaucoma. Lancet 1999;354:1803-1810.
18. Visvanathan AC, McNaught AI, Poinoosawmy D, et al. Severity and stability of glaucoma: patient perception compared with objective measurement. Arch Ophthalmol 1999;117:450-454.
19. Ivers RQ, Cumming RG, Mitchell P, Attebo K. Visual impairment and falls in older adults: the Blue mountains Eye Study. J Am Geriatr Soc. 1998;46:58-64.
20. Sherwood MB, Garcia-Siekavizza, A, Meltzer MI, Hebert A, Burns AF, McGorray S. Glaucoma's impact on quality of life and its relation to clinical indicators. A pilot study. Opthalmology 1998; 105:561-566.
21. Gutierrez P, Wilson MR, Johnson C, et al. Influence of glaucomatous visual field loss on health-related quality of life. Arch Ophthalmol 1997;115:777-784.
22. Parrish RK, Gedde SJ, Scott IU, et al. Visual function and quality of life among patients with glaucoma. Arch Ophthalmol 1997;115:1447-1455.
23. Lee PP, Budenz DL, Chen PP, et al. A multi-center, retrospective study of resource utilization associated with severity of disease in glaucoma. Presented at the Annual Meeting of the American Academy of Ophthalmology (AAO), October 20-23, 2003, Orlando, FL.
24. Collaborative Normal-Tension Glaucoma Study Group. Comparison of glaucomatous progression between untreated patients with normal-tension glaucoma and patients with therapeutically reduced intraocular pressures [published correction appears in Am J Ophthalmol. 1999;127:120]. Am J Ophthalmol.1998;126(4):487-497.
25. Mao LK, Stewart WC, Shields MB. Correlation between intraocular pressure control and progressive glaucomatous damage in primary open-angle glaucoma. Am J Ophthalmol. 1991;111(1):51-55.
26. Heijl A, Leske C, Bengisson B, et al. Reduction of intraocular pressure and glaucoma progression: results for the Early Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268-1279.
27. Lichter PR, Gillespie B, Musch DC, et al. Intraocular pressure as a predictor of visual field loss in the Collaborative Initial Glaucoma Treatment Study. American Academy of Ophthalmology Annual Meeting; Anaheim, Calif, Nov 16-19, 2003. Abstract 33.
28. Lichter PR, Musch DC, Gillespie BW, et al. Interim clinical outcomes in the Collaborative Initial Glaucoma Treatment Study comparing initial treatment randomized to medications or surgery. Ophthalmology 2001;108:1943-53.
29. Fremont AM, Lee PP, Mangione CM, Kapur K, Adams JL, Wickstrom SL, Escarce JJ. Patterns of care for open-angle glaucoma in managed care. Arch Ophthalmol. 2003 Jun;121(6):777-83.