What's New for Your Practice from the AAO Annual Meeting
NEW PHACO SOFTWARE
Sovereign WhiteStar v.6.0 software, which offers surgeons improved flexibilty and control for removal of all lens types, is now available. Features include:
- WhiteStar technology in single-burst, multi-burst and continuous-burst modes as well as continuous and long pulse
- linear foot pedal control of four WhiteStar duty cycles
- ability to name MMP modes and increase flow rates to 50 cc/min
- option to control IV pole height from the foot pedal.
"The new 6.0 WhiteStar software for the Sovereign allows variable duty cycles along with linear phaco power in footswitch position 3. This simultaneous dual control permits more finesse and therefore is even more energy-sparing," said Lisa Arbisser, M.D., who has been using the software in her practice.

ThinOptX has filed its IDE application with the FDA to begin U.S. clinical trials of the ThinLens. The company also:
- unveiled its injector, which is currently reusable and delivers the IOL rolled rather than folded. An injector will be distributed with every lens by the middle of this year. The injector is made of Teflon instead of plastic, which means no viscoelastic needs to be applied to the instrument, a time- and money-saving feature for surgeons.
- reported that the ThinLens is now manufactured in an expanded power range of 10D to 35D. The U.S. list price is expected to be $400 to $500.
- is exhibiting a prototype of its phakic anterior-chamber lens this month at the ESCRS winter refractive meeting in Barcelona. The 100-micron-thick lens has a unique vault system and fixation method. For example, 50 microns of material resides in the eye's angle for extra security.
In addition, surgeons are wrapping up four controlled studies of the ThinLens' accommodative capabilities and are reporting accommodation of 1.75D to 2D.
UPDATES FOR THE ANTERIOR AND POSTERIOR SEGMENTS
In line with its strategy of providing perimetry and imaging products to analyze both structure and function in the detection and management of glaucoma, cataract, refractive and retinal disease, Carl Zeiss Meditec introduced several new products, including:
- VISUCAMlite, which allows digital capture and comparison of fundus images and fluorescein angiography. Doctors can also obtain images using integrated infrared beams at times when pharmacologic pupil dilation is a problem. VISUCAMlite is a stand-alone system with new software but can also be integrated into the entire practice network.
- version 4.0 software for the Stratus OCT, which features a second normative database for macular thickness analysis and was expected to receive FDA approval after the first of the year. In addition, several presentations at the meeting addressed the application of OCT technology to the anterior segment to improve refractive surgery care.
- Glaucoma Progression Analysis software as an upgrade to the Humphrey Field Analyzer II and II-i instruments. The software is designed to differentiate true glaucoma progression from random variability in visual field testing. It adds objectivity to clinical decision-making by applying knowledge gained from the Early Manifest Glaucoma Trial, where the actual test-retest variability of hundreds of glaucoma patients was measured.

Accutome exhibited its new AccuPen tonometer, which is available to practitioners this month. The instrument can store up to nine IOP readings at a time and it gives a confidence level for each reading. The AccuPen doesn't require calibration, and it's ergonomically designed for comfortable measurement-taking.

Heidelberg Engineering debuted its FDA-approved handheld CCT pachymeter, the IOPac. The IOPac is based on Palm technology and specially designed for glaucoma care. It automatically corrects IOP calculations using one of five built-in formulas that reflect established data on the correlation between central corneal thickness and Goldmann tonometry. Physicians can create up to three custom formulas for individualized correction and store up to 1,000 patient records.
The IOPac was developed by Portable Ophthalmic Devices Inc. of Bettendorf, Iowa, and also features built-in USB and infrared for data transfer, printing and storing.
Heidelberg has also enhanced the Retina Module for its HRT II by adding the capability for lateral (x-y) distribution of edematous areas and also a 9-zone trend analysis feature for tracking disease progression and monitoring treatment. The trend analysis feature is based on the Early Treatment of Diabetic Retinopathy Study staging system.

ScienceBased Health added Optic Nerve Formula to its line of ocular nutraceuticals. Many conditions, including ischemic neuropathies and glaucoma, can compromise optice nerve health. The new formula supports normal vascular and nerve function, promotes ocular blood flow, and increases protective antioxidant intake. It contains a blend of Ginkgo biloba; antioxidants such as vitamins C and E, alpha lipoic acid and n-acetyl cysteine; omega-3 fatty acids (DHA, EPA); magnesium; B-vitamins; taurine; and bioflavonoids.
"It is increasingly evident that oxidative processes play an important role in a variety of ocular diseases, including macular degeneration and glaucoma," said Ron P. Gallemore, M.D., Ph.D., Assistant Clinical Professor, Jules Stein Eye Institute, UCLA School of Medicine. "Optic Nerve Formula is an evidence-based formula with a scientifically sound rationale."
SOFTWARE HELPS GUIDE PRACTICE INTERNET STRATEGY
The recently released BetterStats software package from Einstein Industries provides practices with real-world knowledge about their Web sites' marketing trends and tools to build infrastructure for a winning Internet strategy.
BetterStats converts standard Web traffic logs into a marketing knowledge base. It reduces the cost of Web log management and extracts data necessary for measuring the efficiency of Internet media. The software is useful for information technology departments dealing with large amounts of data acquisition and also for marketing and sales departments charged with monitoring advertising efficiency and customer profiling.
INSTRUMENT APPROVALS AND UPGRADES
Nidek Inc. received FDA approval for two instruments, the GYC-1000 Green Laser Photocoagulator and the US-1800 Pachymeter & A-Scan Ultra Sound combination unit.
The compact and portable GYC-1000 delivers a solid-state green laser with 1.7 watts of energy, plugs into any standard wall outlet, and weighs less than 15 pounds. It allows for several delivery options, including slit lamps, indirects and endophotocoagulation.
The US-1800 Pachymeter & A-Scan Ultra Sound combo includes the latest IOL power calculation software, a touch-screen panel, and is portable for use in remote locations. It can be combined with the Nidek US-2500 for an A/B Scan combination unit.
Also launched was the 6 mega-pixel stereo fundus cam era, the 3-Dx Digital Camera. The unit, ideal for assessment of glaucoma, macular edema and other retinal diseases, allows the operator to take repeatable simultaneous stereo color digital images of the optic disc, fundus, and external eye with just one-shot.
The company also announced added features and capabilities for other instruments, including:
- cup-to-disk ratio measurement for the NAVIS Information System Software
- point-spread function diagnostic data and analysis for the OPD-Scan autorefractor/wavefront analyzer/topographer.
AMD TREATMENT RESULTS PRESENTED
Two-year data on Alcon's investigational treatment for wet AMD, anecortave acetate (Retaane 15 mg Depot), presented at the meeting adds to and confirms the previously reported 12-month findings, according to investigators.
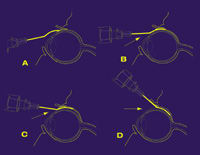
|
|
| The Retaane 15 mg Depot delivery method, called posterior juxtascleral depot, allows Retaane to be administered onto the outer surface of the back of the eye with a curved, blunt-tipped cannula, which does not pierce the eyeball. This approach helps to prevent intraocular infection and retinal detachment, which are the most common side effects of injecting therapeutics directly into the eye. It also allows diffusion of the drug over months, decreasing the number of applications necessary. |
Investigators randomized 128 patients with wet AMD 50 years or older to receive Retaane 15 mg, anecortave acetate 3 mg suspension, anecortave acetate 30 mg suspension or placebo. (55 patients were available for 24-month follow-up) In the Phase II/III trial, 73% of all patients with wet AMD treated with Retaane 15 mg Depot had stable or improved vision after 2 years based on logMAR testing. That compared with the 47% of patients who received placebo (p=0.035). In the subset of patients with predominantly classic lesions, 80% had stable or improved vision after 2 years compared with 42% of the placebo group.
There was no clinically relevant difference in the incidence of cataracts or treatment-related IOP increases between Retaane 15 mg and placebo. All treatment-related adverse events occurred with similar frequency across treatment groups and were described as non-serious, mostly mild and transient and did not interrupt treatment.
Alcon has also launched three additional Phase III trials of Retaane. One will compare its effectiveness with verteporfin for injection (Visudyne) in 500 patients diagnosed with predominantly classic wet AMD. Twelve-month efficacy data from this trial will be used to complete the filing of the FDA New Drug Application.
Two others will evaluate Retaane treatment every 6 months vs. placebo in patients with advanced dry AMD who are at risk of progressing to wet AMD. The studies will include approximately 2,500 patients. The FDA has "fast-tracked" Retaane for this indication.








