at
press time
Alcon AMD Drug Shows Efficacy in Study
Two-Year Data Is Positive for Retaane.
Alcon, Inc. says 24-month results from a Phase II/III clinical trial of its investigational new drug, anecortave acetate for depot suspension (Retaane 15 mg), demonstrate that long-term use of the drug preserves vision, prevents severe vision loss, and inhibits lesion growth in patients with wet AMD.
|
|
|
|
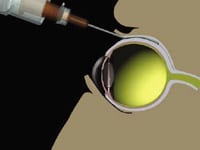
Retaane is delivered directly behind the macula with a specially designed
cannula. |
|
According to Alcon, Retaane 15 mg works by slowing or stopping the growth of new blood vessels, thus producing less leakage and retinal damage. In the study, after 24 months, 73% of patients treated with Retaane 15 mg had stable or improved vision based on a standard AMD vision test. This was significantly higher than the 47% of patients in the placebo group who had stable or improved vision. A total of 55 patients completed the entire 24-month study in which patients were randomly chosen to receive one of three concentrations of anecortave acetate (3 mg, 15 mg or 30 mg) or a placebo.
Investigators said patients treated with Retaane 15 mg had no increase in growth of the classic component of the subfoveal choroidal neovascular membrane between months 12 and 24, and the total lesion size remained stable over this period. Additionally, in a sub-group of patients with the more aggressive, predominantly classic CNV lesions, no patients treated with Retaane 15 mg had vision loss of greater than 6 lines, compared with 23% who had a vision loss of this magnitude in the placebo group.
"This new data adds to and confirms the previously reported 12-month findings, which showed that a 15 mg Retaane treatment has the potential to effectively preserve sight and deliver tangible benefits to wet AMD patients," said Henry L. Hudson, M.D., F.A.C.S., of Tucson, Ariz., an investigator in the study.
Over the course of the study, patients received Retaane every 6 months in a new, in-office procedure known as posterior juxtascleral depot (PJD). During PJD, the investigator, with the help of an assistant, uses a specially designed cannula to place the suspension onto the outer surface of the back of the eye directly behind the macula. This method of delivery allows the drug to diffuse across the sclera and choroid into the macular portion of the retina over a period of 6 months. Alcon says, in contrast, other investigational therapies are less patient-friendly, requiring frequent direct injections into the eye.
Alcon has now launched a 500-patient Phase III trial to directly compare the effectiveness of Retaane 15 mg against verteporfin for injection (Visudyne) in patients diagnosed with predominantly classic wet AMD. Visudyne therapy is currently the only FDA-approved treatment for wet AMD.
Ophthalmic Industry Women
Form New Career Development Group
Its Aims Include Networking and Professional Growth.
Three women executives are spearheading the formation of a new organization whose goal is to facilitate networking opportunities and promote career development for professional women in the ophthalmic industry.
|
|
|
|
Tamara Church of Heidelberg Engineering |
|
"At our larger meetings, I couldn't help but notice that the men in our industry seem to know each other better, and do far more networking," says Tamara Church, who handles professional relations for Heidelberg Engineering. "I felt that women in the industry -- especially women newer to ophthalmology like me -- didn't have the benefit of that kind of camaraderie."
Earlier this year, Church approached Jane Aguirre, publisher of the American Academy of Ophthalmology magazine, Eyenet, and Jan Beiting, president of Wordsmith Consulting, and together they formed Ophthalmic Women Leaders (OWL).
"I think OWL is very appropriate because our aim is to share wisdom and experience by exchanging information, providing educational opportunities, and encouraging mentorship for women who aspire to leadership positions in our industry," says Church.
One of OWL's first initiatives was to create an advisory board, which includes such high-profile ophthalmic industry executives as Elizabeth Davila, chairman and CEO of VISX, Inc.; J. Michelle Glossip, CEO of Duckworth & Kent; Adrienne Graves, Ph.D., CEO of Santen, Inc.; James V. Mazzo, president and CEO of Advanced Medical Optics; Marguerite B. McDonald, M.D., of the Southern Vision Institute; and Kate Tiedemann, president of Katena Products, Inc.
"We're going to concentrate first on providing networking opportunities by holding two receptions per year, one at AAO and one at ASCRS," says Dr. McDonald. "We'll grow from there."
Dr. McDonald says that as the only ophthalmologist on the advisory board, she can act as a conduit between the ophthalmologists and the women executives.
The first OWL reception will be held during the AAO meeting on Nov. 14 from 5:30 to 7 p.m. in the Rancho Las Palmas Room of the Anaheim Marriott Hotel. RSVP to owlbox@cox.net by Nov. 7.
MCAC: Cover Visudyne for Occult AMD
CMS Will Make Reimbursement Decision.
The Medicare Coverage Advisory Committee (MCAC) has recommended that Medicare reimbursement of verteporfin (Visudyne) be expanded to include patients with the occult form of wet AMD. Medicare already offers coverage for Visudyne therapy in AMD patients with predominantly classic lesions.
The MCAC recommendation will now be considered by the Centers for Medicare and Medicaid Services (CMS), which makes decisions on reimbursement.
The recommendation came after the American Academy of Ophthalmology (AAO) made a presentation to MCAC outlining its preferred practice pattern for the use of Visudyne in certain patients with the occult form of AMD.
"The Academy believes that photodynamic therapy with verteporfin in select patients with subfoveal choroidal neovascularization with occult but no classic choroidal neovascularization is a vital and important treatment for this potentially blinding disease," said retinal specialist George Williams, M.D., of the Academy's health policy committee. Dr. Williams made the AAO presentation to MCAC.
The AAO said its recommendation is supported by evidence obtained from descriptive studies, case reports, reports of expert committees, and expert opinion such as consensus by the Academy's preferred practice pattern panel.
REFRACTIVE SURGERY UPDATE
Expedited review. Ophtec USA and American Medical Optics (AMO) said their Pre-Market Approval (PMA) application for the Artisan/Verisyse phakic IOL has been accepted by the FDA and assigned expedited review status. The PMA application is for the correction of myopia in phakic eyes ranging from -5D to -20D. The lens, which attaches to the periphery of the iris, will next be reviewed by the FDA's Ophthalmic Devices Panel. If it receives final FDA approval it will be marketed by AMO in North America under the trade name Verisyse.
LaserSight files Chapter 11. LaserSight, the Florida-based developer of laser vision correction equipment, filed for Chapter 11 bankruptcy protection in September. The company, which reported a $2.4 million loss in the first quarter of 2003, had a $10 million order to sell equipment to a Chinese company but said it fell behind in filling the order because of a lack of cash to pay suppliers. LaserSight CEO Michael R. Farris left the company to pursue other interests in August.
Appointed. Charline Gauthier, O.D., Ph.D., MBA, has joined IntraLase Corp., which develops femtosecond laser technology for use in vision correction surgery, as vice president, Research, Development and Corporate Affairs. Prior to joining IntraLase, Dr. Gauthier served as vice president and general manager of Alcon Laboratories' Orlando Technology Center. She had previously held the post of chief operating officer of Summit Autonomous Technologies, Inc.
"Dr. Gauthier's demonstrated leadership skills, depth of general and international management experience, and technical background bring valuable contributions in supporting the technology and corporate development of IntraLase," said Robert J. Palmisano, the company's president and CEO.
LADARWave software recall. Alcon has conducted a company-initiated recall of the software in 157 LADARWave Custom Cornea units due to a software error that could lead to refractive surgeons retrieving patient data incorrectly. Alcon said the glitch is in the "sort by" function. The company has sent out a safety alert to all LADARWave owners asking users not to sort patient data by "first name" or by "last name." Searching by the patient's full name or medical record will yield the correct information, the company says.
"We're basically asking LADARWave owners not to use certain sorting features and to use other options instead," said Mary Dulle, Alcon's director of corporate communications.
Alcon said the owners of all 109 domestic units affected by the recall have acknowledged the safety alert and taken appropriate precautions.
TLCV custom procedures. TLCVision, a provider of laser vision correction services, said custom LASIK procedures represented about 25% of total volume in its most recent 3-month reporting period. The company said future bookings suggest that momentum for custom treatment is gradually building.
Epikeratome approved. CIBA Vision has received FDA approval for the Centurion SES EpiEdge epikeratome. The EpiEdge, which is used in a new refractive procedure called Epi-LASIK, is a sub-epithelial separator that produces an epithelial sheet. This eliminates the need for alcohol in refractive laser procedures such as LASEK and PRK, resulting in faster healing and less pain for patients, according to CIBA Vision.
Epi-LASIK was developed by Ioannis Pallikaris, M.D., Ph.D., a Greek ophthalmologist who was also instrumental in introducing LASIK.
IN THE NEWS
CIBA Surgical business. CIBA Vision, the eye care unit of Novartis, said it's considering strategic alternatives for its Surgical business. One option currently being studied is the sale of that unit.
CIBA Vision's Surgical business encompasses ownership or licensing of such products as the Ex-PRESS mini glaucoma shunt; the PRL and Vivarte phakic IOLs, the Centurion SES microkeratome, and VisThesia, a viscoelastic with anesthesia that's now available in Europe.
"While the Surgical team has developed a portfolio of highly promising technologies, we do not have the scale or necessary presence in the market to fully capitalize on them," said Joe Mallof, CIBA Vision CEO. "We believe that the value of our Surgical products and technologies can be better realized by a company focused on maximizing their growth potential. This will allow us to focus on our core lens and lens care businesses."
HPR marks 25 years. Health Products Research (HPR), which partners with its panel of ophthalmologists to track trends from contact lens dispensing to cataract surgery, is celebrating its silver anniversary this year. As a leader in pharmaceutical market research, HPR has been conducting ophthalmic studies since its inception.
"The detailed information physicians give us about their practices, surgical procedures and dispensaries has provided the industry basis for new R&D, products and innovations," says Rebekkah Carney, HPR manager of client services.
Ophthalmologists interested in being on the panel can call (877) 471-5743.
Generic glaucoma drug. The FDA has approved Falcon Pharmaceuticals' brimonidine tartrate ophthalmic solution 0.2%, the generic equivalent of the glaucoma medication Alphagan. Falcon, a manufacturer of generic drugs for the eye and ear, is an affiliate of Alcon, Inc.
Shark liver oil for AMD? Genaera Corporation of Plymouth Meeting, Pa., said it has achieved positive early results in a Phase I/II study using squalamine delivered intravenously to treat wet AMD. Squalamine, a derivative of shark liver oil, has also been seen by some as a potential treatment for a wide variety of cancers.
AMO exceeds goals. Completing its first year as a publicly owned company, American Medical Optics (AMO) was able to exceed its initial sales and earnings estimates, resulting in about a 50% gain in the company's stock. Among AMO's first-year achievements were a restructuring of the company's balance sheet, an agreement to purchase a contact lens care solutions manufacturing facility in Spain, and a step-up in R&D spending.
"AMO ended 2002 with an R&D investment of 5.5% of revenues," said Jim Mazzo, AMO's president and CEO. "By the end of this year, we expect that to increase to between 6 and 6.5%; and over the coming years we expect that to be around 8% of revenues. This commitment will allow us to continue to deliver innovative products to practitioners and maintain our leadership position within the industry."
Eyetech IPO. Eyetech Pharmaceuticals, which is partnering with Pfizer to develop Macugen, a treatment for wet AMD and diabetic macular edema, will offer shares to the public in a $100 million stock offering. Macugen is currently in Phase III clinical trials.
Glaucoma screening. Alcon Inc. said it has provided free glaucoma screenings to more than 1,000 at-risk African-Americans since it launched Project Focus earlier this year. The screenings are conducted at local churches, community centers and cultural events.
Prevent Blindness Day. Santen was once again the lead sponsor this summer for Prevent Blindness Day at San Francisco's Pac Bell Park. The program enables hundreds of local children to receive eye screenings while also attending a San Francisco Giants baseball game.
Dry eye treatment. The FDA has accepted Inspire Pharmaceuticals' New Drug Application (NDA) for approval of its dry eye treatment diquafosol tetrasodium (INS365) eye drops in a 2% preservative-free solution. INS365 stimulates receptors located on the ocular surface and inner lining of the eyelid, enhancing the secretion of water, salt, mucin and lipids -- key components of natural tears.
The NDA submission includes data from one Phase II and two Phase III studies involving more than 1,200 dry eye patients. The FDA is expected to rule on the application in late December.
Appointed. Bausch & Lomb has named Paul G. Howes senior vice president and president of the company's Americas Region. Howes joins B&L from Merck, where he most recently served as vice president of the Mid-Atlantic Business Group.
Partnership. Synergetics, a manufacturer of microsurgical instruments, will market Quantel Medical's range of photocoagulators to operating rooms and selected office markets in the United States and Canada. Synergetics will sell and service the Viridis green laser, the Viridis Twin dual wavelength green and infrared laser, and the Iridis diode laser.
FTC investigation. The Federal Trade Commission has begun an investigation to determine whether Allergan, its partner Syntex Pharmaceuticals, or anyone else is monopolizing the market for Acular, a drug intended to reduce eye pain after corneal refractive surgery. Generic drug maker Apotex has accused Allergan of attempting to monopolize the market for Acular in the United States.
On the board. Alcon has named former airline executive Thomas G. Plaskett to its board of directors. Plaskett had previously served as CEO of both Pan Am Corporation and Continental Airlines.