Ramping Up for Custom Ablation
Part 1 of 3
Ride along as one practice takes wavefront
for a test drive.
BY LANNY B. HALE, M.D.
In ophthalmology today, as in all of medicine, we are faced with a continuous stream of technologic advancements. Just as we adapt to the last breakthrough, the next one is upon us.
But how do we know that the latest gizmo really works the way we're told it does? Take the case of our latest breakthrough: the application of wavefront technology to the human eye to drive a refractive treatment. The hardest thing for me to believe is that these new treatments may not require nomograms, and the data from the wavefront diagnostic devices, including manifest refraction, may be fed directly into the laser. As a surgeon who helped develop some of the early laser nomograms and who would never operate on an eye without a cycloplegic refraction or two, this sounds scary.
I've seen convincing presentations on the repeatability of wavefront measurements, and papers have been written on the accuracy of wavefront refractive data compared with conventional refraction and autorefraction, but this one I'm going to have to check out for myself. And this time, I can.
Building Our Knowledge Base
About 3 months ago, we purchased a wavefront unit (a WaveScan because we use the VISX laser system). We made a wavefront analysis the initial test in our standard-LASIK refractive workup, and we began testing everyone, pre-op patients, post-op patients, dilated, undilated, in every situation we thought it might provide useful information.
By putting the wavefront technology through these paces and consulting with experienced users, we've begun to build the knowledge base we'll need when the laser platform we've invested in receives FDA approval for custom procedures. Although the various wavefront instruments are different in some ways, and no standards between instruments exist, we can give you an idea of the issues we're looking into, which should be helpful regardless of the particular instrument you will purchase.
Key questions and what we're finding so far:
What data can we obtain? A great deal. What we're using at this point is:
- objective refraction in hundredths of a diopter
- pupil size at which the measurement was taken
- point-spread function, which is a representation of what the patient would see when looking at a point source of light, like a street light
- an acuity map, which represents all aberrations displayed together (This map is typically dominated by the lower-order aberrations and should appear symmetrical.)
- a map that reflects only the higher-order aberrations (This map can take on almost any appearance because we each have a unique set of higher-order aberrations.)
- higher-order aberration percentage, which relates the lower-order aberrations to the higher-order aberrations and displays their relative contributions
- a higher-order aberration root mean square (RMS) value (The RMS value gives us an idea of the seriousness of the measured aberrations.)
How does wavefront data correlate to a patient's vision? We've learned that on our unit, a higher-order aberration RMS value of 0.5 microns or greater is likely to correlate with visual degradation. Taking this number into account along with the fact that conventional LASIK tends to increase higher-order aberrations, we're delaying treating these patients until we are performing custom procedures.
How can we use the higher-order aberration percentage number? At the most recent national meetings, experts have advocated delaying treatment on patients whose higher-order aberration percentage is greater that 20% of their overall aberration amount. We've had only a couple of patients who fall into this category in the past 3 months and have recommended that they wait until we are performing custom LASIK to undergo their procedures.
Patients with elevated higher-order aberration percentages are most likely those with low amounts of lower-order aberrations (myopia, hyperopia, and astigmatism) thus making even the normal amount of higher-order aberrations appear elevated by comparison, those who have reduced preoperative BSCVA, those with somewhat decentered corneal apices, or those who have had previous LASIK. We could argue that a conventional treatment now can always be followed by a wavefront treatment later, but if the result turns out to be hyperopic or mixed astigmatism, it could be a long time before those approvals are obtained.
Can we use wavefront data to refine our current standard refractions? By obtaining a wavefront reading before our own manifest refraction, we have the option of using the objective information to adjust our subjective refraction in a effort to refine it and the resultant BSCVA. This has proven valuable in terms of astigmatism as so many patients reject their cylinder, and an objective measurement is helpful to support our retinoscopy.
We have experienced isolated cases in which the wavefront refraction is as much as 2 diopters more minus than a known endpoint, which indicates that it can be difficult to control accommodation in some patients. We're trying very hard to keep patients from focusing on the fixation light by telling them to stare through the light rather than staring at it. But when we experience such a variation, we analyze all available data, including standard cycloplegic refraction, cycloplegic wavefront data, manifest data, and the effectiveness of the current correction. Because a significant hyperopic result is always an undesirable effect, we always take the conservative path and use the least myopic refraction.
We've also made subtle adjustments in our standard treatments based on wavefront data in cases with odd variations between cycloplegic and manifest refractions.
How does pupil size affect the wavefront refraction? The eternal problem of night-vision complaints has become more understandable as we've analyzed wavefront refractions. After we take a wavefront meaurement, we can adjust the pupil size used to calculate the refraction. (Our unit has a maximum capture size of 6 mm.) When we've used the photopic and scotopic pupil readings and adjusted the wavefront accordingly, we've seen some interesting effects.
I, for example, have a photopic pupil size of 3 to 4 mm and a scotopic pupil size of 7 mm. My photopic refraction is essentially 1D, but at 6 mm the myopia increases to 1.6D. Because my scotopic pupil is 7 mm, we can assume an even greater increase in myopia in a truly dark situation. Patients without much of a difference between their photopic and scoptopic pupil sizes don't present much of a dilemma, but what do we do with patients like me?
Currently, some surgeons are suggesting that we choose an intermediate pupil size, such as 5 mm in my case, and make an appropriate adjustment. I feel that this approach is safe in younger patients with strong accommodation and a greater likelihood of further myopic progression, but not in the presbyopic population. Therefore, today, we use this information to make small adjustments in younger patients' treatment plans and as an additional way to educate older patients on their likely surgical results.
What can wavefront data tell us about post-op patients? The point-spread function, as mentioned previously, uses math to reproduce the theorectical effect of an eye's aberrations on a point of light. We can adjust this to concentrate on all aberrations or only higher-order aberrations. But because a patient experiences all the aberrations together, we set this to reflect the total effect of all aberrations. This has proven useful for corroberating postoperative reports of ghosting, glare, and other distortions.
|
DON'T MISS |
|
Part Two, February issue: A look at how surgeons are planning for wavefront-guided LASIK as it pertains to their case volume and their net revenue. Part Three, March issue: How custom procedures will affect patient flow. |
|
Much More to Learn
So now you know what we know after our initial experiences with wavefront technology. And you may still be wondering if perhaps we're kidding ourselves if we believe wavefront-guided treatments are significantly better than conventional ones. After all, we're talking about ultrafine adjustments made to optical systems to remove the smallest of imperfections in an effort to create the optimal image. Furthermore, minute amounts of tissue are involved in higher-order aberrations, we induce aberrations by creating a flap and performing our current laser treatments, and aberrations change with age and accommodation. But according to the data that's been submitted to the FDA, the results of custom treatments are outstanding.
No one has all the answers yet, but without questions there can be no answers and without experience it's hard to ask the right questions. We'll forge ahead into the world of this latest technologic advance because our patients want the latest, best possible treatments, and we're committed to providing them.
Dr. Hale is in private practice in Brookfield, Wis., and Scottsdale, Ariz. He's an associate clinical professor of ophthalmolgy at the Medical College of Wisconsin and the founder of the Advanced Vision Correction Foundation, LLC. You can contact him at (262) 789-9029, (480) 661-1600 or lbhale@attglobal.net.
ALLEGRETTO FROM WAVELIGHT
Standard Results as Impressive as Custom
BY CHARLES R. MOORE, M.D., F.I.C.S.
Advances in laser technology have the potential to enable highly successful LASIK outcomes, including those for high myopia. Smoother ablations and larger optical zones made possible by scanning technology are yielding positive outcomes with a higher degree of predictability. Excellent visual outcomes are being reported from all 11 U.S. centers participating in clinical trials with the Allegretto scanning excimer laser (WaveLight).
Impressive visual acuity gains at 1-year follow-up (See "UCVA" at right and "BSCVA" on the next page.) and equally impressive decreases in night vision problems (i.e., glare, halos) have been observed. It is likely that the laser's ability to create a prolate cornea with its optical benefits is largely responsible for the outstanding objective and subjective visual outcomes reported in these trials.
|
|
|
|
Post-op uncorrected visual acuity data on 739 eyes from all 11 U.S. centers participating in clinical trials with the Allegretto scanning excimer
laser. |
Preserving Tissue with Technology and Technique
Because scanning lasers, with their larger optical zones, may remove more tissue per diopter of ablation than broadbeam lasers, the need for tissue preservation is especially critical. Tissue preservation is a function of both technology (the laser itself) and technique (thin/custom flaps). Positive outcomes require that a surgeon choose a laser treatment profile that takes the least amount of tissue possible, and not all scanning lasers are "created equal."
A comparison of FDA protocol data reveals some notable differences among the scanning excimer lasers being studied. The Allegretto has an active tracker with a response time of less than 6 milliseconds, and delivery is up to 4 times more rapid than with some of the other scanning lasers. (200 Hz vs. 50 Hz, respectively). The Allegretto enables ablation of a much larger optical zone while taking substantially less tissue per diopter because of the 0.95-mm flying spot. Tissue preservation was a prime consideration in the design of the Allegretto. Nomograms based on wavefront-based algorithms (rather than derived from Munnerlyn formulas) give the laser the flexibility needed to safely treat up to 14D of myopia and, in the process, achieve impressive results with standard or custom ablations.
Most unique, and clinically significant, is the Allegretto's ability to maintain or create the cornea's natural prolate curvature. The normal prolate (more steep centrally than peripherally) cornea becomes oblate (flattened center) when treated with PRK or LASIK performed with most other lasers. It is this conversion from prolate to oblate that is partially responsible for the low-light or night-vision problems so common with standard LASIK. Because this transformation is averted with the Allegretto, problems related to glare and halos are virtually eliminated.
|
|
|
|
Data on post-op changes in best spectacle-corrected visual acuity on 739 eyes from all 11 U.S. centers participating in
clinical trials with the Allegretto laser system. |
|
Outcomes Exceed Expectations
To date, I have treated 160 eyes with up to 14D of myopia and up to 6D of astigmatism in the WaveLight Allegretto FDA protocol trials. At 1-year follow-up, 96.4% of eyes have best-corrected visual acuity of 20/20 or better, and 75% have uncorrected visual acuity of 20/16 or better. 50% of the eyes have gained 1 or more lines of acuity; 25% gained 2 or more lines; and none have lost 2 or more lines of vision. These findings have been validated by all 11 U.S. protocol investigators.
An unanticipated, and extremely positive, finding was the 0.5% enhancement rate reported by all 11 investigators. This rate is 2 times lower than that achieved with any other lasers available in the United States. Furthermore, patient satisfaction screening revealed a significant decrease in night vision/low-light vision complaints, with none of the patients treated reporting glare or halos. This critical finding can be attributed to the laser's ability to maintain a prolate cornea.
Mean flap thickness in the series of 160 eyes was 118 microns (range: 60-185 microns), and 100% were left with the 250-micron basement needed to prevent iatrogenic corneal ectasia.
Customizing Flaps
Successful, complication-free LASIK outcomes depend not only on the laser, but also on careful preoperative planning, intraoperative decision-making and thin (custom) flap creation and management when faced with thin corneas or high myopia.
Preoperative planning must take into consideration the unique aspects of each patient's orbital anatomy (bone, lid, lashes, conjunctiva, etc.) so that the optimal keratome and surgical approach are employed for each patient. It is especially critical that all decisions made preoperatively be validated with intraoperative pre- and post-flap pachymetry and post-ablation pachymetry. This is the only reliable way to determine the actual flap thickness and residual stroma that can safely be ablated. This information is critical to preventing iatrogenic corneal ectasia, which can occur if a flap is thicker and the residual corneal bed thinner than predicted. Because of the wide variance in industry tolerances, reliance on a keratome can result in large (+/- 50 microns) discrepancies between intended and achieved flap thicknesses. Furthermore, formulas for estimating the amount of tissue removed for a particular correction don't take into consideration factors such as corneal hydration, laser speed, spot size, and optical zone size that affect such decisions.
Finally, while a flap thickness of 160 microns is acceptable for simple LASIK cases within normal treatment ranges (very little tissue to ablate), tissue preservation is paramount for treatment of an eye with even moderate, and especially high, myopia and a thin cornea. A thin (custom) flap is needed to ensure that adequate tissue remains, underscoring the value of a laser designed to preserve as much tissue as possible.
The rationale for custom flaps is especially compelling in light of the irreversible complication that can arise from unplanned thick flaps, and that can be prevented when thin (custom) flaps are carefully made and managed. While most LASIK complications (e.g., incomplete flaps, epithelial abrasions, DLK) can be successfully managed, iatrogenic corneal ectasia can have devastating visual consequences, requiring corneal grafting.
Keys to Excellent Outcomes
Successful standard or wavefront-guided LASIK outcomes can be ensured by careful attention to surgical technique and use of a scanning laser designed for tissue preservation. LASIK procedures performed with the state-of-the-art Allegretto laser are clearly producing excellent objective and subjective visual outcomes, largely because of the laser's ability to maintain the cornea's natural prolate curvature.
The transition from broadbeam to scanning technology can be a smooth one for surgeons who remain vigilant with respect to surgical technique. Thorough preoperative planning, predictably thin (custom) flaps, careful intraoperative decision-making and expert custom flap management are now more important than ever.
Dr. Moore is the director of refractive surgery at the International EyeCare Laser Center in Houston.
ZYOPTIX
FROM BAUSCH & LOMB
Wavefront Plus Full Corneal Analysis
BY STEPHEN SLADE, M.D.
W avefront technology promises superior results both in terms of traditional metrics and in quality of vision for our refractive surgery patients. It also promises to provide us with a way to help patients who have had poor results with previous surgery.
Wavefront aberrometers are able to accurately measure the total power of the eye. This provides surgeons with personalized patient data they can input into the laser to have a customized correction applied to the cornea. The Zywave II Aberrometer, which is part of Bausch & Lomb's custom ablation platform, can even display for patients the difference in the quality of vision they might expect, such as how they might view a star in the sky, with wavefront LASIK compared to conventional LASIK.
While the wavefront device displays the overall power of the visual system, it does not look specifically at the corneal surface that needs to be reshaped by the laser. Thus the Bausch & Lomb Zyoptix Diagnostic Workstation combines wavefront technology with the Orbscan IIz corneal analysis system.
The Orbscan IIz corneal analysis system is able to examine both the front and back surface geographies of the cornea, its full thickness, and pupil diameter to assess a patient's candidacy for this exciting procedure. By displaying these individual influences of the cornea, the surgeon will be able to see how and where the laser will treat the eye and what affect the treatment will have on the cornea.
Maximizing the Optical Zone
Combining wavefront technology with total corneal analysis in one workstation provides validation to these complementary technologies, thereby adding a fail-safe mechanism to the procedure. Validation of the wavefront data by the Orbscan IIz helps ensure that safe and predictable treatments are programmed into the Bausch & Lomb laser, providing the patient with the best vision correction possible.
Furthermore, Zyoptix is proven to reduce the amount of tissue removed for a given level of correction, particularly in mid to higher levels of myopia. This feature provides two distinct benefits depending on the treatment objective.
First, it is important to optimize the optic zone size for quality results. Thus tissue preservation is essential in order to treat higher myopes or patients with thinner corneas with larger optical zones. Second, because the objective is to improve visual performance beyond standard LASIK, the surgeon ideally would maximize the optical zone to at least 6 mm in order to remove higher-order aberrations and deliver improved visual performance. The Zyoptix system enables surgeons to use the widest treatment zones possible, while preserving the most corneal tissue.
Another major benefit of Zyoptix is that it improves visual performance in real-life conditions. Zyoptix produces the best visual performance results when the optical zone is maximized. With optical zone sizes above 6 mm, fewer high-order aberrations will be induced.
Results from subjective patient questionnaires show that Zyoptix patients do notice a difference in their vision in "real-life" conditions.
To fully appreciate the benefits, it is important to assess outcomes using more than a bright-light Snellen visual acuity measurement. Both objective and subjective vision should be taken into account:
Objective:
- bright light visual performance
- dim light visual performance
- contrast sensitivity
Subjective:
- pre- and post-op patient questionnaires
- quality of vision in dim light
- impact on night driving
- existence of glare and halos using backlit acuity testing (BAT) equipment.
Zyoptix Patient Selection
Optimal results with customized ablation can only be achieved if the surgeon respects the importance of selecting the right patients and adhering to a standardized procedure. Patients who fall into three broad groups will benefit the most from Zyoptix:
- Large (dim light) pupil size of at least 5.5 mm. One can expect a benefit in visual performance with a dim light pupil size of 5.5 mm. The highest visual performance will be achieved with an optical zone size of 6 mm or greater.
- Significant preoperative higher-order aberrations. The extent of higher-order aberrations is now quantifiable with the Zywave software.
- Higher myopes and patients with thin corneas. Whether a surgeon can treat these patients is determined by the ability to ablate a 6-mm optical zone while leaving at least 250µm of residual stromal thickness.
The importance of following a standardized approach is one of the most critical components for success. The most critical aspect of this is the development of a personalized nomogram. Also important is the need to use staff members who are trained in using the Zyoptix system in order to ensure consistency in diagnostic measurements, Zylink calculations, surgical procedure, and data collection, both pre- and post-op
The set protocol for Zyoptix users should include:
- Obtaining a dilated Zywave measurement within +/-0.75D sphere and +/-0.50D cylinder to the baseline subjective refraction. The cylinder must be within +/- 15 degrees but only for cylinder above 0.50D.
- Making adjustments to the PPR values of the dilated Zywave measurement within the Zylink software only according to the surgeon's individually developed nomogram.
Looking at Results
Results of the U.S. Zyoptix clinical trials conducted to date demonstrate excellent results. In the prospective, multicenter trial conducted at three investigational sites, including mine, a total of 340 eyes were treated. This trial included treatment for both sphere and cylindrical components of refractive error. Subjects were followed through 6 months. One nomogram was used throughout the entire study. It is compelling to note that in this trial, an impressive 100% follow-up was achieved.
Preservation of best-corrected vision is a key safety variable for any refractive procedure. Looking at preoperative vs. 6-month postoperative best-corrected spectacle visual acuity, only 13% of eyes reported with 20/12.5 or better VA before surgery, but 46% achieved this high level of visual acuity after surgery. Similarly, 54% saw 20/16 or better before surgery and 88% achieved this level of vision at 6 months. This clearly demonstrates a substantial improvement in best-corrected vision after surgery.
At 6 months, more than 60% of eyes reported with an improvement in their best-corrected visual acuity compared with their preoperative best-corrected acuity. One third (33.8%) reported no change, resulting in more than 94% of subjects who maintained or improved from their best-corrected vision. It is important to note these results were achieved with no nomogram adjustment in the Zyoptix data.
The efficacy ratio, the comparison of best-spectacle-corrected vision before surgery with uncorrected vision after surgery, is a primary metric of any refractive procedure. In the Zyoptix study, after surgery, 78.3% of eyes reported better vision without correction than they did with correction before their LASIK procedure.
The majority of patients reported either no change or an improvement in light sensitivity 6 months post-op vs. pre-op. Patients reported a 37% improvement in light sensitivity vs. pre-op. Patients also reported either no change or 21% improvement vs. pre-op for glare. The most significant findings were reported on difficulties with night driving. 50% reported no change and 40% reported an improvement in difficulties with night driving. At 6 months following treatment, 98.8% of patients reported satisfaction with their surgery. No patients were dissatisfied. Also:
- 92% of eyes saw 20/20 or better with no correction in place
- 78% achieved uncorrected vision that was better than their pre-op best-corrected vision
- 97% either maintained or improved upon their pre-op contrast sensitivity
- 99% reported they were "satisfied" with their surgery.
- 91% reported they were "very" satisfied.
The Zyoptix data show the system to be highly effective for the correction of both spherical and cylindrical refractive error, providing excellent visual acuity with and without correction, superb quality of vision and very satisfied patients.
Dr. Slade, of Houston, Texas, is the medical monitor for the Zyoptix clinical trials.
CUSTOMEYES FROM LASERSIGHT
Success for Virgin and Post-Op Eyes
BY JACK T. HOLLADAY, M.D.
Over the past 3 years, LaserSight has built its custom ablation platform around the desire to create a solution for improved refractive outcomes for ALL patients. This custom ablation solution (CustomEyes) is focused on providing improved primary treatment outcomes, quantitatively and qualitatively, as well as addressing the need to re-treat previous surgical cases that exhibit imperfections such as decentered ablations, central islands, small optical zones and irregular astigmatism. These imperfections typically relate to poor visual acuity, night vision glare, halo, double vision, reduced contrast sensitivity and other visual dissatisfaction. The LaserSight CustomEyes platform includes three components: a comprehensive diagnostic measurement device, a custom ablation planning software, and a 3-D precision scanning microspot laser:
|
|
|
|
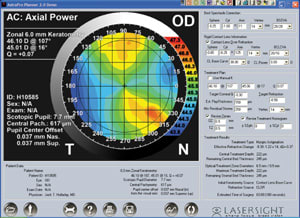
The AstraMax stereotopographer has three cameras that allow the height and curvature of each point to be calculated
independently of any other point. With conventional monocular topographers, every point on the surface is calculated from the point just inside the one being calculated. The result is
cumulative error, which is eliminated with stereo. |
|
AstraMax, the diagnostic tool, employs stereoscopic principles and a polar grid target to provide improved corneal measurement accuracy including curvature and elevation in corneal topography. Corneal pachymetry is measured using an optical imaging method. Scotopic and photopic pupil sizes and their location relative to the corneal apex can also be measured. Among the many measurement capabilities, the instantaneous measurement (less than 0.2 seconds) of the scotopic pupil size and pupil location relative to the apex of the cornea and the elevation of the anterior cornea are essential and unique to the LaserSight custom ablation solution. Multiple exams of the patient eye can be analyzed and averaged to generate an exported data file for the planning software.
AstraPro, the custom ablation planning software, imports the AstraMax data including anterior elevation, scotopic and photopic pupil size, pupil center location relative to the location of the apex, and corneal pachymetry. It also requires subjective manifest refraction data as input. Manifest refraction takes into account the subjective aspect of human vision to provide a starting point for laser treatment calculation.
The custom ablation algorithm used in AstraPro incorporates the AstraMax data to produce the target refractive correction as well as a desired (prolate ellipsoid) corneal shape. A prolate cornea reduces higher-order aberrations that normally accompany laser vision correction: asphericity (Q) describes the shape of the ellipsoid. A prolate surface has a negative Q value; a value of 0.26 corresponds to a typical prolate cornea. In a typical primary cornea, the surgeon usually plans preservation of the pre-op asphericity, or the normal prolate surface of Q = 0.26. In previous surgical cases that exhibit visual imperfections, the corneal asphericity is positive or oblate; the goal through treatment with AstraPro is to restore the prolate shape of the cornea.
|
|
|
|
The AstraPro software screen showing a patient's irregular astigmatism and preoperative parameters. |
Variable Optical and Treatment Zones
AstraPro custom treatments are planned using a customized optical zone based on the scotopic pupil size. Utilizing a variable optical zone and treatment zone conserves corneal tissue without compromising the quality of treatment. The planning software displays the custom ablation profile and simulated post-op topography. Upon surgeon evaluation, the treatment plan is exported to a disk and the disk is used by the scanning laser to perform the treatment as planned.
In developing the CustomEyes platform, it was LaserSight's belief that the majority of the aberrations of the visual system are attributed to irregularity of the cornea, and therefore can and should be corrected on the cornea. In the unlikely event of lenticular irregularity, the CustomEyes solution recommends a set of rigid gas permeable (RGP) contact lenses to eliminate the irregularity of the cornea and identify the true cause of the visual aberrations. After applying the RGP lens, an over refraction is done to determine the best-spectacle-corrected visual acuity. If in such case, the BCVA is not 20/20 or better, we can conclude that there are additional causes to the visual problem in addition to the cornea. This patient would be excluded from treatment with the CustomEyes platform or the expectations for the patient with such a treatment would be addressed preoperatively.
|
|
|
|
The AstraPro treatment screen showing the same patient's (in image above left) pre-op parameters, ablation plan and desired postoperative result in height data. Notice the difference in the appearance of the irregular astigmatism on a height map vs. the power map (above left). |
|
The final element of the CustomEyes platform is the surgical tool itself: the 3-D scanning precision microspot, AstraScan Custom Laser System. The AstraScan laser leads the industry with the smallest spot, lowest fluence and finest resolution. This 3-D scanning system provides the surgical tool needed to accomplish the precision ablation defined by the AstraPro planning software. An advanced minimum-latency video-based eye tracker, also an integral part of the system, ensures the precision placement of the ablation to accomplish the desired target shape of the cornea.
Phase-One Results
LaserSight recently completed phase-one beta trials of the CustomEyes platform, the results of which have been outstanding. Both primary eyes and surgical eyes were enrolled for the trial. Of the 36 enrolled eyes, 9 were surgical enhancement eyes, 26 were primary eyes and 1 was unclassified. The initial clinical outcomes reported by Dr. Aleksander Stojanovic in Norway at 1 month include the following:
- 34 of the 36 eyes (94%) obtained post-op prolate shape
- post-op UCVA for all eyes is 50% 20/15 or better and 89% 20/20 or better
- post-op BCVA is 90% 20/15 or better for all eyes
- 80.5% of all eyes gained one or more lines of BCVA postoperatively
- post-op UCVA for eyes with pre-op BCVA of at least 20/20 are 55.2% with 20/15 or better and 93.1% with 20/20 or better.
The results are encouraging and exciting because the improvements were obtained for both virgin eyes and post-surgical enhancement eyes. More than 80% of all eyes gained one or more lines of BCVA postoperatively. This suggests that the philosophy behind the algorithm and the argument for a prolate surface works well. The high percentage of eyes that achieved 20/15 or better UCVA suggests that the custom ablation technology and platform are effective.
Dr. Holladay is the medical director for LaserSight's CustomEyes trials.