Practice Watch
Tips and News You Can Use
Can A Microchip Cure Blindness?
Human Trials Are Set to Begin Soon.
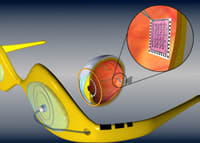
Human clinical trials are scheduled to begin within the next 9 months for an electronic retinal implant that has the potential to restore at least some vision to people blinded by eye diseases such as retinitis pigmentosa and age-related macular degeneration.
|
|
|
|
The key to restoring vision is an electronic microchip that's surgically attached near the retina to stimulate the remaining healthy retinal neurons. |
|
That's the word from the Office of Naval Research (ONR), which is part of a consortium of government agencies that is funding research headed by Mark Humayun, M.D., at the University of Southern California. Dr. Humayun was formerly affiliated with the Wilmer Ophthalmological Institute at Johns Hopkins University.
"Several independent, but cooperative, research efforts have been going on for a number of years, both in the United States and in Europe, to develop an implantable retina to restore some level of vision," says Joel Davis, Ph.D., scientific officer in the Neural and Cognitive Science Division of the ONR. "Dr. Humayun has conducted animal testing using dogs and has now received an FDA protocol to begin tests with humans."
The electronic implant developed by Dr. Humayun's team is designed to work for those diseases where healthy retinal neurons remain intact following loss of function in the eye's photoreceptors. In sighted individuals, these photoreceptors convert images into electronic impulses.
To capture these images electronically, an external camera is first mounted in an eyeglass frame. Images are then converted to electrical signals and transmitted to a flexible silicon biochip surgically attached near the retina. The chip electronically stimulates the remaining healthy cells, which send the signals conveying the image to the brain.
Researchers say studies conducted thus far indicate that the electronic implant technology should be successful in restoring enough sight so that people will be able to recognize faces and read large-print type.
REFRACTIVE SURGERY UPDATE
Cross-cylinder ablation. The U.S. Patent & Trademark Office has issued a patent to Nidek Co. Ltd. for the refractive correction of moderate and high astigmatism with myopia or hyperopia through cross-cylinder ablation. The new technique, developed by Paolo Vinciguerra, M.D., of Milan, Italy, is designed to maintain a prolate cornea that more accurately mirrors the eye's naturally curved shape.
LaserSight patent. LaserSight, which develops lasers and other equipment used in refractive surgery, has received a U.S. patent for a device that illuminates the eye during laser refractive surgery. The company says the illumination device is designed to be used as part of an overall eye tracking system.
Complication rates. A large-scale study of more than 28,500 patients who underwent LASIK in open-access laser facilities during 1999 and 2000 found that 46 of 28,201 (0.16%) of the patients who were treated using a Hansatome microkeratome experienced an intraoperative flap complication, while 21 of 329 patients (6.38%) whose flap was cut with an Automated Corneal Shaper had a flap complication during surgery. Researchers said the difference in intraoperative flap complication rates is "statistically significant."
Procedures plummet. LCA-Vision, a provider of laser vision correction services, reported it performed 10,684 procedures in the 3-month period ending Dec. 31 vs. 16,411 in the comparable period a year ago, a drop of about 35%. The company says a rise in procedure volume in January is encouraging.
Korean approval. South Korea's Food and Drug Administration recently granted conditional approval for the marketing of the STAAR Implantable Contact Lens for myopia in that country.
Assessing the "See Clearly Method"
It Promises to Reduce Dependence on Corrective Lenses.
If you've been listening to the radio recently, you've probably heard the ads for the "See Clearly Method," a vision improvement program that claims to be able to dramatically correct common refractive errors such as myopia, hyperopia, presbyopia and even astigmatism by having individuals perform a series of eye exercises for 30 minutes a day.
Ads for the See Clearly Method, which was developed by two optometrists, say positive results can be achieved in a month or less, enabling some people to end their dependence on corrective lenses. However, no formal research studies are available to confirm these claims.
The See Clearly program is currently being sold to consumers for $210 ($239 for the Deluxe CD-ROM kit). The advertising hints that eye exercises, muscle relaxation techniques, wearing an eye patch, eye massage and undercorrected prescription lenses are all part of the See Clearly regimen. The literature does caution that the program shouldn't be seen as a substitute for professional eye care.
Though the See Clearly ads are designed to create a high level of expectations regarding the potential vision improvement that individuals can derive from the program, ophthalmologists tend to be dismissive.
"There's no way I know of that eye exercises, massage or other manipulation can reduce refractive error or create sustainable improvement in visual acuity," says Eugene M. Helveston, M.D., professor emeritus of ophthalmology at the Indiana University School of Medicine.
And while the American Academy of Ophthalmology hasn't specifically addressed the effectiveness (or lack of effectiveness) of the See Clearly Method, the organization asserts that there's no scientific evidence that vision exercises can correct refractive error.
Here's Four More Marketing Musts
To Help You Market Smarter, Not Harder.
Brad Ruden, president of MedPro Consulting and Marketing Services, says successfully marketing a medical practice isn't just advertising -- it encompasses the entire patient experience.
The key is to market smarter, not harder, he says. The best marketing practices aren't necessarily the ones who spend the most, but those who master a few simple rules. In this final installment of a series of marketing tips, Ruden offers ways to help you improve your practice.
Monitor your competitors. What works for them may be improved upon and work even more effectively for your practice. The key can be in differentiating yourself from the competition or in letting your patients know you offer the same services -- only better.
Associate with a cause relevant to your patients. Pick a cause that's relevant to your practice and patients and then become actively involved. It should be something that you can do well and that could also involve your staff and patients.
For example, you could support the public library's summer reading program. Make sure your involvement is a win-win situation, for you as well as the community. Don't do it with the goal of getting free advertising, do it because it's important and needed. Consider it a plus if any free exposure results.
Exceed the needs of your patients. At a minimum, your patients expect their basic needs to be met. Patients must be seen in a timely manner, treated compassionately and have their questions answered clearly and concisely. But don't just satisfy your patients' needs, get creative and delight them in ways they don't expect.
Utilize the knowledge and experience of your staff. Recognize that your staff is your greatest asset. In most cases, the staff spends more combined time with the patient than you do. If properly trained and provided with well-defined roles, your staff should be able to assist in upgrading almost any area of the practice. They can provide ideas on improving efficiencies to reduce overhead, decrease waiting times, or simply improve the overall experience for the patient.
These steps sound simple, but they're effective. Often, significant improvements can be made just by mastering the little things on a daily basis. And remember, success stems from focusing on the needs of the individual patient.
IN THE NEWS
Acquisition. The Cooper Companies' CooperVision unit has agreed to purchase Biocompatibles Eyecare, the contact lens business of Biocompatibles International. Biocompatibles Eyecare had sales of about $70 million last year.
Partnership. Allergan and EntreMed, Inc. have formed an alliance to develop and commercialize EntreMed's Panzem, a small molecule angiogenic inhibitor that the companies view as a potential treatment for age-related macular degeneration. A key part of the development effort will be the assessment of Oculex Pharmaceuticals' proprietary, biodegradeable drug delivery technology to provide localized administration of Panzem to the back of the eye.
Marketing campaign. CIBA Vision has launched a $20 million North American consumer marketing campaign to promote FreshLook cosmetic contact lenses. The campaign is aimed primarily at females ages 18 to 34.
Xalatan sales. Pharmacia said worldwide sales of its Xalatan glaucoma medication rose to $226 million in the 3 months ending Dec. 31, a 15% increase over the year-ago period. Full-year Xalatan sales for 2001 totaled $818 million, or 18% higher than the previous year.
Approval. Akorn, Inc. has received FDA approval to market Paremyd, a topical mydriatic/cycloplegic combination product indicated for pupil dilation in routine ophthalmic diagnostic procedures and eye exams. Akorn says Paremyd's fast onset decreases waiting time for eye exams, enabling eyecare professionals to better manage patient flow.
Refractive contact lenses. An FDA Ophthalmic Devices Panel has recommended approval of Paragon Vision Science's CRT corneal refractive therapy contact lenses for overnight wear for the temporary reduction of myopia and myopia with astigmatism.
Visudyne growth. Worldwide sales of Visudyne photodynamic therapy for wet AMD grew 127% in 2001 to $224 million. Novartis, the drug's co-developer, says sales were helped by Visudyne's improved reimbursement status and approval of the drug for treatment of pathologic myopia and presumed ocular histoplasmosis. Additional significant sales growth is forecast for this year.
Clear lenses. Transitions Optical is targeting the clear lens market with its Next Generation Transitions 1.50 lens. The company says Next Generation is virtually undistinguishable from regular clear lenses indoors, but gets as dark as sunglasses quickly in an outdoor setting.
Restructuring. Bausch & Lomb is eliminating 700 jobs worldwide in the first phase of a global restructuring. The cuts will be broad-based and will effect all regions and operating units. The company, which recently closed contact lens manufacturing plants in Florida and Spain as part of the restructuring, said it's continuing to seek additional ways to cut costs.
Patent suit. Pharmacia Corp. has sued Pharmaceutical Resources Inc. for infringing on three patents relating to Pharmacia's glaucoma medication Xalatan. Pharmaceutical Resources had earlier announced plans to develop and market a generic version of Xalatan that doesn't infringe on Pharmacia's patents.
New CFO. STAAR Surgical has named John C. Bily as its chief financial officer. Bily was previously vice president and controller, worldwide operations, for Allergan.
Gerber management. Gerber Scientific Inc., a leader in optical lens processing, has named George M. Gentile as its new chairman and Marc T. Giles as president and CEO. Michael J. Cheshire, the company's former chairman and CEO, has resigned to pursue other interests.
Appointed. Association Expositions & Services (AE&S), organizers of International Vision Expo and EyeQuest, has named Eileen Baird as vice president and show manager for AE&S' International Vision Expo/EyeQuest portfolio of events. Baird brings more than a decade of trade show experience to International Vision Expo and EyeQuest.
Discount prescriptions. Novartis Ophthalmics has launched a discount prescription program for low-income Medicare recipients. The company says the first-of-its-kind program, called Novartis Care Card, is available free of charge to Medicare recipients who qualify. The card will provide discounts of 25 to 40% off retail prices for Novartis eyecare products.
Inspire Cuts Back After Failed Drug Trial
Enthusiasm Wanes for Dry Eye Treatment.
When the stock market closed on Tuesday, Jan. 15, shares of Inspire Pharmaceuticals were trading at $15.51.
By the end of the next day, Inspire shares were changing hands at $4.15, representing a loss of about 70% of total shareholder value in just one day. By early February, the stock had fallen even further.

The dramatic drop in Inspire's stock price was caused by an announcement from the company that its highly touted dry eye treatment, INS365 Ophthalmic, had proved no more effective than a placebo in its first Phase III clinical trial. A second Phase III trial for INS365 is continuing, with results expected by late Spring. Inspire is partnering with Allergan in the development of INS365 Ophthalmic.
Inspire's dry eye treatment is based on proprietary technology designed to stimulate the eye's P2Y receptors to activate an individual's mucosal defense mechanisms.
While Inspire says its top priority continues to be further evaluation of the INS365 Ophthalmic program, the failure of the initial Phase III trial is a major blow to the company. Inspire recently slashed its operating budget by 25% and cut back on its ambitious drug development program. The company must now conserve cash as it strives to prove to investors that it can develop treatments with commercial potential.
Inspire said it will complete its Phase I/II trial for its retinal detachment treatment and re-evaluate that program based on study results. The company also has put on hold two other non-ophthalmic drug development programs.
"It's critical to prioritize and focus our efforts toward generating value-creating events in our top programs," said Gregory J. Mossinghoff, Inspire's senior vice president and chief business officer. "This will provide a clear focus and allow us to aggressively drive our near-term opportunities."








