News & Notes from the AAO Meeting
In the February issue of Ophthalmology Management: glaucoma-related news from the AAO meeting
New dry eye therapy
|
|
|
|
CIPTA custom treatment of
decentration. |
|
Allergan launched Refresh Liquigel lubricant eye drops, which combine the strength of a gel with the convenience of a drop for patients with persistent dry eye symptoms.
Tests showed a 52% decrease in combined corneal and conjunctival staining after 30 days of use. A sponsor-masked evaluation of 500 patients showed high overall patient satisfaction with Refresh Liquigel, and 61% of patients reported that they needed to use Refresh Liquigel less frequently than they use their current artificial tears. Also, 90% of patients said their vision was normal, not blurry, within 5 minutes of use.
Custom ablation platform complete
LaserSight has unveiled the new AstraScan Custom Laser System, which was introduced to the international market at the AAO in New Orleans. The AstraScan's features include a new, advanced video-based intelligent eye tracker, a redesigned optical delivery system, and an interface to the CIPTA custom ablation planning software.
|
|
|
|
Liquigel drops have a proprietary blend of molecular weights. |
CIPTA was introduced into international clinical use in 1996, and since that time 22 refractive surgery centers, performing more than 15,000 procedures per year, have been licensed to perform custom ablations using the CIPTA software and LaserSight's excimer laser systems.
LaserSight said the CIPTA custom treatments using its excimer system have demonstrated efficacy, safety, predictability and stability, and results of CIPTA custom treatments have been published in peer-reviewed journals and presented at major ophthalmology venues throughout the world.
LaserSight will provide the CIPTA software on an annual license and/or a per procedure fee basis, and users of current versions of the LaserScan LSX can purchase an AstraScan upgrade to their systems that will facilitate use of the CIPTA planning software and effective execution of the precise ablation treatments.
"Treating patients with irregular astigmatism and retreating patients with decentered ablations from previously performed laser refractive procedures demonstrates the power of the CIPTA software when combined with LaserSight's laser system," said Guiseppe D'Ippolito of Ligi Technologie Medicali, which developed the software.
Current users of the international version of the LaserScan LSX can now purchase an upgrade package that includes all of the new features of the AstraScan. The company will make the AstraScan, AstraScan upgrades and CIPTA available to U.S. customers once all regulatory requirements are fulfilled.
Phaco technology upgrade
Allergan announced the availability of the WhiteStar Technology upgrade for its Sovereign phaco system. The upgrade transforms traditional ultrasound, while providing surgeons the efficiency, control and flexibility they want.
The micropulses of WhiteStar Technology put less energy into the eye than traditional ultrasound, while providing full cutting ability and enhanced followability. The micro-rest periods reduce energy into the eye, allow time for the tip to cool, allow for reacquisition of the fragments and enhance the cavitational effect. Exposing the eye to less energy has been shown to produce clearer corneas 1 day post-op, reduce injury and inflammation in surrounding ocular structures, and result in quicker visual rehabilitation and better outcomes.
Lisa Arbisser, M.D., said she's found the system to be effective on any grade of cataract, including those that are 5+ and brunescent. "What makes WhiteStar Technology exceptional is its efficient use of ultrasound, followability and lack of generated heat, which reduce the fluid flowing through the eye and result in less trauma to ocular structures," Dr. Arbissser said. In her first 135 cases, all done with a vertical chop technique, she measured a 1.25-second mean equivalent effective phaco time (EPT), which is the time adjusted for the average percent of phaco power. In other words, the EPT is the time spent as if the system were operating at 100% power. The mean phaco power was 1.4%.
Dr. Arbisser also expects that this type of technology will facilitate new techniques, such as bimanual phaco.
Visco debut in U.S., phakic IOL update
CIBA Vision will introduce UniVisc cohesive viscoelastic in the United States. UniVisc contains 10 mg/ml of sodium hyaluronate, giving it a high molecular weight and high viscosity. Its cohesive property enhances removability.
UniVisc is ideally paired with CIBA Vision Surgical's MemoryLens and PMMA IOLs. It's delivered through a unique syringe and cannula designed to eliminate bubbles and provide a clear view. The company is offering the Lens Plus Pricing Program, which combines UniVisc with both the MemoryLens and PMMA lenses for significant savings.
In other news from CIBA Vision Surgical, the PRL phakic refractive lens has entered U.S. Phase III clinical trials. The PRL is a silicone, plate-haptic, posterior chamber lens. It's foldable and can be implanted through a clear cornea, 3.5-mm incision. It's designed to be independent of any intraocular structural support for fixation; it floats on the surface of the crystalline lens on an aqueous fluid layer, making sizing a less significant issue.
Clinical investigator Louis Nichamin, M.D., has treated seven patients with the PRL. He said all of the patients were seeing 20/20 or better the morning after the procedure.
|
|
New IOL Available this Quarter |
|
Allergan unveiled ClariFlex, its new foldable, second-generation silicone IOL with OptiEdge design. The lens is implanted with The Unfolder Silver-T Implantation System through an unenlarged incision. Release is slow and controlled. The ClariFlex is considered an ideal stepping stone to the Array multifocal IOL. Discussing the ClariFlex, Dr. Randall Olson said studies by Samuelson, Spalton and others show that the second-generation silicone materials used in the ClariFlex lens exhibit high biocompatibility and perhaps significantly less inflammation than acrylic after 3 years. He also noted that second-generation silicone induces more anterior capsular pressure than acrylic IOLs, which could result in a lower PCO rate. Dr. John Hunkeler said he prefers to avoid enlarging his cataract incision for IOL implantation. He especially prefers silicone lenses for post-refractive surgery patients given the greater likelihood for refractive error after previous refractive surgery. If a lens implant exchange is required, it is easier to cut a silicone lens and then remove it through the original unenlarged cataract incision. In regard to capsule clouding, Dr. Hunkeler said he believes we'll see less and less of a difference between silicone and acrylic because of the truncated edge.
The OptiEdge rounded anterior edge scatters light, which can reduce internal reflections; the sloping side edge is designed to direct reflections away from the retina; and the squared posterior edge facilitates 360-degree capsular contact and a discontinuous bend. |
New products, strategic alliance
As part of its plan to showcase its brand strength and diversity, Bausch & Lomb presented several new products (See "25-Gauge System for Vitreoretinal Procedures," on page 65.) and other developments, including:
- The Hydroview one-piece foldable hydrophilic acrylic IOL with bonded PMMA haptics is now available in the United States. The company said the lens material has a water content of approximately 18%, which allows it to naturally co-exist with the fluids in the eye, and that the bonded modified C-loop haptics keep the lens centered and stable.
The FDA cleared the lens for marketing in 1999, but the company delayed its launch while it investigated a small number of reports of opacification attributed to calcification of the optic. To resolve the problem, B&L sought and obtained FDA approval of a packaging change: The gasket of the SureFold delivery system no longer contains silicone material, which was identified as the catalyst for calcium deposit formation. - The company's eye vitamin and mineral supplement Ocuvite PreserVision, which was shown in the NEI's 10-year Age-Related Eye Disease Study to reduce the progression of AMD, will be available on retail store shelves soon. A daily dose (four tablets) of PreserVision contains: 28,640 IU vitamin A (beta-carotene); 452 mg vitamin C (ascorbic acid); 400 IU vitamin E (dl-alpha tocopheryl acetate); 69.6 mg zinc (zinc oxide); and 1.6 mg copper (cupric oxide).
- Bausch & Lomb has formed a strategic alliance with one of the largest third-party administrators of vision benefit plans offered by health plans nationwide, TruVision Inc. Under the agreement, health plan participants will be offered contact lenses, eyeglasses, eye exams and refractive surgery through a network of doctors assembled by B&L. Bausch & Lomb will have access to TruVision's Web-based contact lens ordering, surgical tracking, scheduling and medical outcomes systems.
FDA activity, LASIK MBA Program
Nidek celebrated its 30th anniversary and announced:
- The FDA granted supplemental pre-market approval for the EC-5000 Excimer Laser System to utilize an increased optical zone, 6.5 mm with a 7.5 mm transition zone, during LASIK to treat myopia and myopia with astigmatism.
- The redesigned ConfoScan Confocal Microscope has received 510(k) clearance from the FDA. The new ConfoScan is easier to use, smaller, and offers more integrated features in an "all in one" tabletop box.
The ConfoScan uses digital recording software to examine all layers of the cornea at the cellular level. Images are displayed quickly for review. Physicians can use the instrument to analyze the epithelium, assess corneal health preoperatively, diagnose early signs of keratoconus and dry eye, assess overall endothelial viability, measure flap depth and assess interface healing quality. - The company submitted a 510(k) application for the DC-3300 laser to be used in glaucoma and retinal photocoagulation procedures. The laser is indicated for use in limited, pan-retinal and transpupillary thermotherapy, endophotocoagulation and transscleral photocoagulation, and glaucoma procedures such as trabeculoplasty and iridotomy. It's designed to be used in combination with various delivery systems, including slit lamps, binocular indirects, endoprobes and transscleral probes.
- The company has teamed up with Howard Gottlieb, president and CEO of Eyecare Consultants Ltd., to provide the LASIK MBA Program for its customers. The program aims to increase a practice's LASIK volume, reduce patient acquisition costs, enhance the patient conversion process and increase patient referral percentages through an on-site evaluation, action plan development and implementation and monitoring of the results. To enroll, call Valerie Goodkin at (203) 288-3081, ext. 309.
- A 510(k) application for the Nidek Advanced Vision Information System (NAVIS) was submitted to the FDA for evaluation of its image restoration function. NAVIS is the only software program for ophthalmic data acquisition to incorporate an image manipulation function. This latest addition will allow for the enhancement of captured images, exposing images that wouldn't otherwise be observed, providing better diagnoses.
IOL milestone, product introductions
In addition to presenting the latest data from the CustomCornea clinical trials, (See "CustomCornea Data")
- Alcon announced the 10 millionth Acrysof IOL implant. "We're extremely proud of this historic event and most grateful to our customers that have partnered with us reaching this milestone," said Mike Smith, director of marketing for the cataract group.
- The company also said it is now introducing new products in every category of retina care, from handheld instruments to retinal stabilizing adjunct products to the recent upgrade of the Accurus vitreoretinal surgery system. The latest generation Accurus includes a high-speed side-to-side cutter that operates up to 1,800 cuts per minute.
Hyperopia treatment update
The FDA ophthalmic device panel voted 9 to 1 to grant approvable status to conductive keratoplasty for the treatment of 0.75D to 3.00D of hyperopia. Due to the lack of long-term data at the time the panel made its decision, CK received labeling claims for the temporary reduction of hyperopia. But Refractec said that data presented to the panel showed that, on average, 93% of the total correction was maintained at the 1-year visit.
Medical monitor Marguerite McDonald, M.D., said that the 12-month regression curve for conductive keratoplasty is almost the same as the curves for PRK and LASIK for hyperopia. Refractec said excellent levels of uncorrected visual acuity were achieved at 6, 9 and 12 months postoperatively, and that the FDA target of 85% of eyes at 20/40 or better was achieved from 3 months forward.
PTAMD trial
Iridex Corporation announced that the Executive Committee of the Prophylactic Treatment of Age-Related Macular Degeneration (PTAMD) clinical trial advised investigators that current enrollment is sufficient to detect a clinically relevant difference in the outcomes of the study and that further enrollment is unnecessary.
"This is good news," said study chairman Thomas Friberg, M.D. "We had initially thought the PTAMD trial would require 1,000 patients followed for 5 years and are pleased that the current enrollment of over 600 patients in the bilateral arm will be sufficient to determine the effect of treatment when adequate follow-up is achieved within a year or two from now."
The trial is looking into whether prophylactic laser treatement for patients with dry AMD could preserve visual acuity and/or reduce the risk of disease progression to wet AMD. The treatment used is a Minimum Intensity Photocoagulation procedure, one of several pioneered by Iridex.
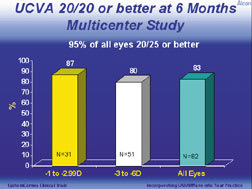
Stephen F. Brint, M.D., F.A.C.S., reported U.S. custom ablation clinical trial results available at 6 months of a multicenter study of 82 myopic eyes treated with the refined Alcon CustomCornea algorithm. (See "UCVA 20/20 or Better at 6 Months, Multicenter Study," below.) The pre-op refractive parameters were:
Dr. Brint explained that Alcon custom ablation efforts to date have been focused on improving quality of vision by reducing higher-order aberrations, and the study data show that the CustomCornea eyes experienced more reduction of higher-order aberrations than eyes treated with conventional LASIK. He cited 6-month data from a subgroup multicenter study of 48 custom and control eyes. (See "Patients with Reduced Higher Order RMS Error," below.) Dr. Brint said that the clinical benefit of treatment of higher-order aberrations could be observed in measures such as contrast sensitivity. In the subgroup study:
Dr. Brint further explained, "The fact that all 82 CustomCornea treated eyes showed a decrease in spherical aberration compared with conventionally treated eyes is highly statistically significant. With conventional LASIK treatments, sometimes you can double or even quadruple higher-order aberrations. The length of our LADARWave trials has allowed us to refine the algorithms to achieve superior quality outcomes. There have been no nomogram adjustments to date to maintain consistency across the study. However, once we are allowed to adjust the nomograms, we predict we'll be able to provide not only a superior quality of vision, but also better acuity levels with no postoperative symptoms." At the Alcon Refractive Wavefront Breakfast, Omar J. Hakim, M.D., FRCSC, of the University of Waterloo, Canada, reported 1-month data on 28 myopic and 8 hyperopic eyes from the international arm of the Alcon custom ablation clinical trials. He said:
Dr. Hakim also reported that 22% of eyes showed a decrease in higher-order RMS error, and the average value of spherical aberration decreased significantly. He also reported his single-center results, in which post-op wavefronts demonstrated significant surgically induced vertical coma. All eyes treated at that site had a superior hinge. This pattern was different from that seen at U.S. sites, where hinges were predominantly nasal. "Soon we hope to modify the algorithm to account for the hinge effect if it can be accurately quantified," Dr. Hakim said. "But we are achieving the same significant reductions in spherical aberrations as the U.S. is experiencing."
|
|
An Update on Reimbursement Policy for Visudyne Therapy |
As part of his update on the status of reimbursement for Visudyne therapy, Jason Slakter, M.D., made the following points: NEW NATIONAL POLICY. Most Medicare decisions related to Visudyne therapy are made by local carriers -- unless a national policy exists. In July 2001, CMS initiated a national coverage policy for photodynamic therapy with verteporfin. Under the policy, Dr. Slakter explained, "Carriers are permitted to cover only predominantly classic lesions." The new policy eliminates a reimbursement problem that physicians faced early on. Because the original clinical trials enrolled only patients whose visual acuities were between 20/40 and 20/200, the initial response from many carriers was that Visudyne wasn't covered for patients better than 20/40 or worse than 20/200. The national policy eliminates that. "It doesn't matter what the baseline vision is because we have evidence that we can achieve stability or reduction of vision loss, regardless of where we started," Dr. Slakter said. He also said that CMS is expected to issue a J code, J3395, for verteporfin therapy on Jan. 26. The new code will replace the Q code that's used now. SUBSEQUENT VISITS. Dr. Slakter explained that there is no limit on retreatment frequency. However, retreatments must be based on continued leakage. And, when a physician decides to retreat a patient, he must obtain a fluorescein angiogram prior to treatment. Also, there is no global period associated with verteporfin therapy. "Therefore," he explained, "If you bring a patient back at 4 weeks, and you want to examine him, and you want to get an angiogram, you may bill for the exam and the angiogram." WHAT ABOUT OCCULT LESIONS? Per the current national policy, local carriers must deny payment for occult disease. However, on May 20, the Vitreous Society submitted a formal request to CMS seeking a change in the policy, which would allow coverage for treatment of unstable occult lesions. (As stated previously, the national coverage policy was released on July 1, but the 2-year data on treating occult lesions from the VIP study hadn't been available to CMS while it was formulating that policy.) The Society cited several points: Medicare does have the authority to cover a procedure that isn't FDA approved; the clinical evidence indicates that verteporfin is safe and effective for occult lesions; and that because no other treatment exists, verteporfin is the standard of care. An Oct. 19 CMS memo stated that the agency was intending to cover occult lesions -- after an appropriate waiting period. Unfortunately, on Oct. 30, CMS announced that it was reconsidering its decision to cover. "So, we're waiting on an updated decision, which we'll probably get in January 2002," Dr. Slakter said. "But that means we'll have no final word until at least the middle of next year." IF YOU TREAT OCCULT LESIONS. While, at this point, physicians will not be reimbursed for treating occult lesions, they can elect to treat. "I'm pleased with the 2-year data on occult lesions at this point, so in spite of the fact that we don't have FDA approval for the occult-only group, I may as a treating physician, not as part of a study group, elect to treat these patients," Dr. Slakter said. "But you have to understand then that it's an out-of-pocket expense for them. Therefore, prior to treatment, you must obtain an advance beneficiary notice so you can charge the patient. And even though you won't be reimbursed, you must submit a claim for your Medicare-covered patients, and that claim must indicate that you've obtained an ABN." Dr. Slakter also reminded attendees that they must be able to support their claims. "You'd better have, in the chart, your angiogram, your interpretation of it, and your diagnosis." NON-AMD LESIONS. Medicare contractors are permitted to cover non-AMD indications. "We do have FDA approval for histoplasmosis and myopic degeneration," Dr. Slakter said. "So there's a valid rational based on clinical trials and FDA approvals to expect that our local carriers should pay for patients with CNV if it's due to those causes and is subfoveal. Submit your claims to your carriers, request a fair hearing if necessary, and provide as much information as possible. Show them that these indications are FDA-approved. That carries the weight of gold."
|
|
WaveScan-Guided Ablation Results; Mixed Astigmatism Approval |
Dr. Colman Kraff presented 6-month data on 19 patients from a study of WaveScan-guided LASIK. The study's pre-op parameters were: mean age 34 ±7 years; mean sphere -1.9 ±0.6D; mean cylinder +0.3 ±0.4D; and mean SE -1.7 ±0.7D. No nomogram adjustment was made, and the post-op target was emmetropia. "The mean sphere was a little lower than in the multicenter trial," Dr. Kraff explained. "This was somewhat intentional because we had no basis for comparison. We were guided only by the WavePrint System." Dr. Kraff went on to say that the majority of myopes are low myopes, "so maybe we can use some of the information we're garnering here to help alleviate some of their fears and help get them excited about refractive surgery." The study also made use of the PreVue lens technology, which allows patients to see the type of result they can expect from treatment. "It also gives the doctor a validation that the result will be very close to the intended result, so there's less guesswork," Dr. Kraff said. (One of the requirements for this study was that patients be able to read, with each eye, a minimum of three letters on the 20/16 line with the PreVue lens.) Dr. Kraff said results were consistent at 1, 3 and 6 months. (See "Uncorrected Visual Acuity".) The study also compared pre-op BSCVA with post-op UCVA. "This is a new category we're now looking at," Dr. Kraff said. "We're really concentrating on the uncorrected visual acuity." (See "Pre-op BSCVA to Post-op UVCA".) He cited a typical patient from this group, a 2D myope, for whom the root mean square was reduced by about half. "So we had some reduction in the overall higher-order aberrations in this patient," he said. "No eyes lost any more than three letters of BSCVA, not three lines, but three letters, of BSCVA. One patient had a significant epithelial defect, and interestingly, at 6 months, her uncorrected visual acuity was 20/12. So once the epithelium had smoothed out, her vision was outstanding post-operatively. "These results set a new standard. 74% of the patients had a UCVA of 20/16; 58% had a post-op UCVA better than their pre-op BSCVA; 100% of patients had a manifest refractive spherical equivalent within .5D of emmetropia; and 90% were within .25D." Following the same study design, Dr. Kraff also treated 14 eyes bilaterally with higher refractive errors. Mean sphere was -5.2D; mean cylinder was +0.6D; and mean spherical equivalent was -4.9D. At 1 week, 67% of those patients achieved 20/16 UCVA, and 100% achieved 20/20. No eye lost more than 2 letters of BSCVA; 6 eyes experienced mild photophobia; 4 eyes experienced mild dryness. VISX also announced that the FDA approved the use of its STAR Excimer Laser Systems for the treatment of mixed astigmatism. The approval covers LASIK treatment of naturally occurring mixed astigmatism, where the sphere and cylinder have opposite signs and the magnitude of cylinder (Ð 6.0D at the spectacle plane) is greater than the magnitude of sphere.
|
Attendees Share What They Learned
"At this year's AAO meeting, I broke tradition as a cataract and refractive surgeon and attended the symposium on macular degeneration and nutrition. There, Dr. Mark Tso presented the Zimmerman lecture on the pathologic basis of AMD at the cellular level. The presentation greatly aided my understanding of the subject. It was very convincing to see the 5-year data and the overwhelming support for antioxidants and zinc in preventing the progression of AMD."
-- John Jarstad, M.D., Federal Way, Wash.
"Two papers that were presented on November 13 are particularly important to highlight:
- The Collaborative Initial Glaucoma Treatment Study (CIGTS) Interim Outcomes Report with Up to 5 Years of Follow-up. Senior author: Paul Lichter, M.D.
- Initial Quality-of-Life Findings with Up to 5 Years of Follow-Up in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Senior author: Nancy K. Janz, Ph.D.
"These papers represent the interim results of one of the major clinical trials in glaucoma. As clinicians, we are always wondering whether newly diagnosed glaucoma patients should be treated initially with medications or surgery. This multicenter, randomized prospective trial has determined after the first 5 years of the study that both medical and surgical therapy result in stabilization of visual fields. Also, the quality of life, although initially depressed in the surgical patients is about the same after 5 years of follow-up. The authors do not recommend changing our current practice based on these results.
"This is important because these study results differ from the Moorfields study performed several years ago. In that study, newly diagnosed glaucoma patients were randomized to either medical therapy, laser surgery, or trabeculectomy. The patients who underwent trabeculectomy were more stable long-term. However, this study was performed prior to the introduction of some of the newer medications we have now, and the population was different from a diverse American population.
"CIGTS is an American study that included a diverse population. Some of the newer medications were used, and the patients in the medication group could also be treated with laser trabeculoplasty. Thus, CIGTS is more applicable to our current practice.
"The take-home message is that one should continue to consider medical therapy as initial treatment in most cases, that is mild to moderate cases. In my own practice, I will follow a single drop or two medications with laser trabeculoplasty. However, in severe cases, one should continue to be more aggressive."
-- Eve J. Higginbotham, M.D., Maryland
"I attended the free-paper session chaired by Robert Weinreb, M.D., and Roger Hitchings, FRCS, which included comparison of the efficacy of travoprost in black vs. non-black patients compared with latanoprost or timolol. The paper presented by Peter Netland, M.D., provided discussion of evidence we have seen before that Travatan appears to be more effective in blacks vs. non-blacks, and this can be very important when treating this higher-risk patient population.
"Dr. Mark Latina's discussion of selective laser trabeculoplasty as primary therapy for glaucoma also has a potentially big impact on the way that we treat glaucoma. Selective laser trabeculoplasty provides a therapeutic effect similar to that seen with argon laser trabeculoplasty but without the permanent damage that argon laser trabeculoplasty creates. The procedure is well tolerated and relatively easy to perform. The advantage of laser therapy is that it can provide intraocular pressure lowering with minimal risk and cost to the patient. Dr. Latina's paper substantiated what we saw in the selective laser trabeculoplasty phase III clinical trials.
"The information in these sessions helped to confirm that using some of the newer medical agents in at-risk populations, as well as novel laser therapies, will only continue to improve the effectiveness and the quality of care we deliver. By knowing the likely risks and success rates of our medical, laser and surgical therapy, we can provide better care to our patients."
-- Robert Noecker, M.D., Arizona
"I attended the State Affairs Forum because we are all affected substantially by our individual state environments. It is important for me to learn about the political issues that face other states because these same issues may face my state soon.
"During the forum, Michael Brennan, M.D., presented awards recognizing individuals who have made significant contributions to state affairs activities. Members of state societies discussed legislative victories and creative ways to protect patients and to promote ophthalmologists. Joe Gagen, J.D., presented an interactive and humorous talk on advocacy strategies.
"The message that I took home was that we all can learn creative methods to sincerely interact with our legislators to affect positive change. All ophthalmologists should promote and safeguard patient safety and our medical profession. Participating in state affairs is a critical method of preserving and improving our practice of ophthalmology."
-- Abdhish R. Bhavsar, M.D., Minnesota
"In addition to the scientific sessions, I enjoyed the Association of Visual Artists art exhibit, which consisted predominantly of paintings and photographs created by ophthalmologists.
"Ophthalmologists have a great deal of artistic talent. There were a number of oil, watercolor and acrylic paintings, as well as photographs. This year, I submitted "Nirayudh," a photograph of my baby son, who appears blue, like Krishna, because of a fluke during the developing process. My husband, Abdhish R. Bhavsar, M.D., submitted "Rajasthan," a photograph of a silhouette of camels taken in the desert in Northern Rajasthan, India. He also submitted "The Jungle," an abstract acrylic on canvas painting.
"It was wonderful to see so many creative works from ophthalmologists! The message for me was to continue to be creative because without passion and creativity, we would simply be automatons! We should do everything we can to support this exhibit every year."
-- Mary A. Bhavsar, M.D., Minnesota
|
25-Gauge System for Vitreoretinal Procedures |
Dr. Eugene de Juan Jr. spoke about his experience with the Bausch & Lomb 25-Gauge Entry Site Alignment (ESA) device, which was recently approved by the FDA for use with the Millennium TSV25 microsurgery system and high-speed vitrectomy cutter. The 25-gauge instruments are designed to facilitate posterior segment surgeries through smaller incisions with fewer steps and no need for infusion-line sutures. To gain access to the posterior chamber, the surgeon sterilizes the globe of the eye and uses the insertion trocar to insert the 25-gauge cannula through both the conjunctiva and the sclera. The general surgical approach then becomes: insert cannulas, perform procedure, remove cannulas. "The 25-Gauge Entry Site Alignment and vitrectomy system, when used transconjunctivally, greatly facilitates the ease and speed of common vitreoretinal procedures," Dr. de Juan said. He said that the 25-gauge system cuts in half the time it typically takes to open, perform a vitrectomy (not counting membrane peeling) and close, from approximately 35 minutes to approximately 19. Dr. de Juan has done 60 cases with the system and has found it to be safer than conventional systems. "Eyes heal almost instantly," he said. Dr. de Juan said that the system could be used in the majority of vitreoretinal cases. |