Practice Watch
Tips And News You Can Use
Ophthalmologists Lose in Senate, House Races
Opponents Prevail Over Drs. Cooksey, Brown and Sumers.
For the first time in 6 years, there won't be an ophthalmologist in Congress in 2003.
That's because John Cooksey, M.D., the conservative and popular Louisiana Republican who had already served three terms in the U.S. House of Representatives, gave up the House seat that political observers said he could have easily retained in order to run for the U.S. Senate.
Dr. Cooksey faced an uphill battle from the beginning, taking on a respected incumbent, Mary Landrieu. But the Cooksey camp received a major setback in August when the Republican National Committee endorsed and funded the Senate bid of Suzanne Haik Terrell, the state commissioner of elections. With Louisiana Republicans split, Dr. Cooksey received only 14% of the vote on Nov. 5, while Terrell got enough votes to earn her way into a runoff election with Landrieu in December.
Two other ophthalmologists were defeated in their bids for seats in the U.S. House of Representatives:
In a hotly contested Pennsylvania campaign, Melissa Brown, M.D., a Republican who ran as a fiscal conservative but a liberal on social issues, made a respectable showing against the Democrat incumbent, Rep. Joseph Hoeffel. Running for a House seat that represents parts of heavily Democratic northeast Philadelphia and more affluent suburban areas, Dr. Brown garnered 47% of the vote to Hoeffel's 51%. It was Dr. Brown's second unsuccessful run for Congress.
And in northern New Jersey, Anne Sumers, M.D., formerly a moderate Republican who became a Democrat, received only 38% of the vote in her race against conservative Republican Scott Garrett. Both candidates were seeking to replace longtime House member Rep. Marge Roukema, a moderate Republican who chose not to seek another term.

|

|

|
| John Cooksey, M.D. | Melissa Brown, M.D. | Anne Sumers, M.D. |
From the AAO
News from the recent American Academy of Ophthalmology Meeting
Custom ablation approval. Alcon's LADARVision laser system is the first to receive FDA approval for performing wavefront-guided custom LASIK. The system has received clearance for myopia from 0.75D to 7D and less than .5D of astigmatism.
"For the past 3 or 4 years, refractive surgeons have been focused on improving the safety of LASIK, in areas such as reducing microkeratome-related problems, improving nomograms, and dealing with dry eye," said Dan Durrie, M.D. "Now custom treatments are our move into the area of quality of vision. Patients are telling us that's what they want."
Dr. Durrie said the LADARWave system has been shown to reduce problems with contrast sensitivity and night driving by inducing far less spherical aberration than traditional LASIK performed with the same system. Also, he said he's comfortable treating patients who have more than .5D but less than 1.5D of astigmatism, as long as their manifest refraction is within a diopter of their wavefront refraction.
"In my patient base, 40% to 50% of those interested in LASIK fall within that range," he said. "Candidates, of course, must have a normal corneal thickness. Large pupils aren't often a concern because the treatment zone for this custom procedure is 9 mm. And, in patients who want monovision, I don't recommend the procedure for the monovision eye, but I especially like it for the distance eye."
Lumenis launches four products. Lumenis continued to strengthen its presence in the growing market for smaller ophthalmic lasers by introducing four new products at the AAO meeting. The company says it spent more than $27 million on R&D in the past year, focusing on developing space-saving laser systems suited for use in offices and ASCs. Products launched include:
- The Novus Varia, a three-color, portable, diode-pumped solid-state photocoagulator. The yellow wavelength enables ophthalmologists to work within the macula nearer to the fovea. The red wavelength enhances effectiveness in treating through vitreous hemorrhage. The green wavelength can be used for basic photocoagulation
- The Novus TTx, a small, 810nm diode-based laser designed for use in transpupillary treatment for AMD, retinopathy of prematurity and thermal treatment of ocular melanomas. (This product is awaiting FDA 510K approval)

- The Novus Spectra, a diode-pumped, solid-state, portable photocoagulator for the treatment of a variety of retinal conditions, is the most powerful green laser that Lumenis manufactures for ophthalmology. Its "true" 50-micron SureSpot Laser Link allows ophthalmologists to be certain they're treating a specific area with safety and efficacy.
- The Selecta Duet is the first system to provide a laser for selective laser trabeculoplasty (SLT) for the treatment of glaucoma, in combination with a YAG laser for the treatment of secondary cataracts and other YAG procedures. SLT reduces IOP without causing thermal damage and scarring to the trabecular meshwork.

Lumenis has also entered into strategic alliances with Ellex Medical of Australia and Moria S.A. of France that are expected to broaden the worldwide distribution of Lumenis ophthalmic laser products.
Successful CK launch. Refractec, Inc. announced that its limited U.S. rollout of Conductive Keratoplasty (CK), a nonlaser procedure for treating hyperopia, resulted in 3,860 procedures performed by 54 ophthalmologists.
"The demand for CK has grown at a very rapid pace, compared to past vision correction procedures," said Mitchell B. Campbell, Refractec's president and CEO. "In the first 6 months, CK-trained physicians have averaged 15 procedures per month."
Based on this level of acceptance, Refractec has stepped up plans for a national rollout of CK. The company estimates that the number of ophthalmologists offering the procedure will top 250 by the end of this year, and 350 by the end of the first quarter of 2003.
In clinical trials, nearly 95% of CK patients reported being "satisfied" to "extremely satisfied" with their visual outcomes. The procedure provided a restoration to normal vision in 93% of patients, according to clinical trial data collected at 24 months post-CK. Though the FDA initially labeled the procedure as temporary, data analysis indicates that patients, on average, are experiencing no significant refractive change between visits at 12 months and 24 months.
Panel: Microphaco worth considering now. During an AMO-sponsored panel discussion, moderator Randall Olson, M.D., asked a large gathering of surgeons whether they were interested in performing bimanual microphaco. Ninety percent, as recorded by the electronic Audience Response System, said yes. Most of those who answered no said they were waiting for small-incision IOLs to become available before employing microphaco in their practices.
"That's certainly a significant issue," Dr. Olson said, "but we're finding that the technique has advantages even without the lenses." In addition to the promise of better patient outcomes, he and other panel members cited exquisite wound control; a stable chamber; the ability to switch instruments to handle any situation, including subincisional cortex; enhanced flow; and that irrigation assists in maneuvering lens fragments toward the phaco needle, rather than pushing them away.
Panel members pointed out that making the capsulorhexis in a microphaco procedure with forceps is challenging, and surgeons are better off using a needle or 23-gauge forceps, which are now available. It was noted that several companies are coming out with instrumentation especially suited for microphaco.
Whether to use a 0-degree or a 30-degree phaco tip was also discussed. Dr. Olson said he uses a 30-degree tip because it's difficult to get past Descemet's membrane with a 0-degree tip. Howard Fine, M.D., also said he prefers to use a 30-degree tip, but with the bevel down. William Fishkind, M.D., said one argument for using a 0-degree tip is that it directs the cavitational energy directly ahead of it. "There's no question where it's going," he said.
Whether microphaco is safer than standard phaco has yet to be definitively proven, Dr. Olson said, but he said he has no doubt that "eventually we'll all be going to microphaco."
Diabetic retinopathy stages. The international Diabetic Retinopathy Task Force presented consensus recommendations for a simplified classification system of the stages of diabetic retinopathy (DR). The Task Force noted that the complexity of the Early Treatment Diabetic Retinopathy Study staging system, and the development of different grading systems in several countries, made it clear that a single, practical classification system that could be used worldwide was needed. The Task Force said the new system, when implemented, will facilitate communication between healthcare providers.
Using the new classification system, which defines five stages ranging from no DR to proliferative DR, practitioners will be able to distinguish different degrees of DR and initiate appropriate treatment.
New TheraTears products. Advanced Vision Research will introduce two new products under its TheraTears brand in January. TheraTears Liquid Gel is a lubricant eye gel for nighttime use, or for dry eye patients who need a longer-lasting protective film during the day. TheraTears Nutrition is a fortified flaxseed oil supplement for dry eye relief. TheraTears Nutrition contains eicosapentaenoic acid (EPA)-enriched flaxseed oil in softgel capsule form. Advanced Vision Research says this formulation decreases meibomitis, augments the oil layer of the tear film, and stimulates tear secretion.


HRT II cornea module. Heidelberg Engineering announced that it plans to introduce an enhancement to its HRT II analyzer in 2003, pending FDA clearance. The Rostock Cornea Module (RCM) is an attachment to the HRT II with specially designed software for anterior segment analysis. The RCM will provide ophthalmologists with in vivo histology of the cornea, a reproducible image in 64 cross sections, with up to 800x magnification down to 1 micron resolution. The RCM is expected to be useful as a pre- and post-LASIK assessment tool, providing an automatic count of keratocytes, as well as a density count of epithelial and endothelial cells.
Low-vision education. Lighthouse International is introducing a new continuing education course for eyecare professionals called Low-Vision Reimbursement and Practice Management.
This 1-day course will be given by qualified Lighthouse instructors at Lighthouse headquarters in New York City on Feb. 27.
Low-Vision Reimbursement and Practice Management is designed to help eyecare professionals understand the new Medicare provisions regarding low-vision rehabilitation and other practice management essentials. It will help them integrate low-vision care into their practice, covering the key practice management issues involved in bringing low-vision services to patients. The course is designed both for eyecare professionals who are interested in exploring all the options available to them for helping low-vision patients, and also for those who desire to make a commitment to providing low-vision services within their own practice.
For more complete information on the time, location and content for this course, you may call (800) 829-0500, or e-mail education@lighthouse.org, or visit the www.lighthouse.org Web site.
Nidek to appeal. In a speech given at the AAO meeting, Hideo Ozawa, the founder and president of Nidek, said the company will appeal a recent federal court decision that Nidek infringed on two excimer laser patents originally held by Summit Technology, Inc. Summit was acquired by Alcon, Inc. in 2000.
Ozawa said Nidek has won each of its past patent disputes and expects to prevail in this one. He also denied rumors that Nidek might exit the excimer laser business and asserted that the company is "here to stay" in terms of supporting existing excimer laser products and developing new ones.
In his speech, Ozawa listed the five guiding principles that serve as the foundation for the company's business philosophy. These principles include customer focus, product quality, innovation, product diversity and using the company's extensive knowledge base to expand its market position.
Laser launched. Carl Zeiss Meditec AG introduced the MEL 80 excimer laser, its first new product since the merged company was created in July. The MEL 80 offers greater precision and faster treatment of refractive errors than its predecessor, the MEL 70 G-Scan, and is approved for sale in Europe and parts of Asia.
New LASIK tool; pupil size and night vision. VISX released its Star S4 laser upgrade, which gives surgeons the capability to create PreVue lenses for the first time. The lenses, which are ablated with a planned LASIK treatment, allow patients to subjectively assess their potential result from either a standard or a custom procedure prior to undergoing surgery. Poor candidates can be screened out before they become unhappy patients.
For the surgeon, ablating a PreVue lens also serves as a check of the laser and the measured wavefront. If a patient has minimal higher-order aberrations, he often will not experience improved visual acuity through the lens.
|
|
|
|
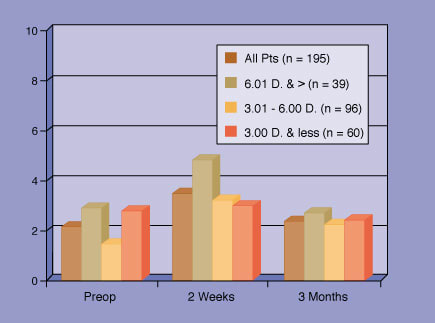
Subjective Symptoms Patients with a scotopic pupil size larger than 7 mm who underwent conventional LASIK with the VISX Star S3 laser (6.5 mm optical zone blended to 8 mm) reported an increase in their night driving problems 2 weeks post-op, but a decrease to near pre-op levels at 3 months. |
|
Among other results achieved with the VISX S3 laser, using conventional, nonwavefront, ablations, three surgeons presented new data that show a lack of correlation between patients' pupil size and night vision complaints.
In one such study, John Doane, M.D., performed myopic/astigmatic LASIK on 195 eyes of 109 patients, all of who had scotopic pupil sizes of more than 7 mm (up to 9.68 mm) as measured with the PupilScan II (Keeler). Patients were treated with a 6.5-mm optical zone and a blend zone out to 8 mm. Refractive data pre-and post-op were taken with the WaveScan aberrometer set at a 6-mm aperture. Patients rated their subjective vision wearing glasses or contact lenses before surgery and again after surgery on a Visual Analog Scale questionnaire.
The mean pre-op spherical equivalent was -4.34D; mean pre-op cylinder was .69D. Dr. Doane said 119 eyes have undergone their 3-month post-op evaluations, and he described their refractive results as excellent: The mean spherical equivalent was -.25.
At 2 weeks post-op, patients rated their problems with glare, halos, starbursts and night driving as worse than before surgery. But at 3 months post-op, they reported that their complaints decreased; all subjective indices decreased to near pre-op values. "That's what we want to see," Dr. Doane said.
Also of interest from this study, he said, was that higher entering refractive error did not correlate to greater subjective complaints or to a greater increase in post-op RMS values than lower entering refractive error. And, he said, "No patients have said they wished they hadn't had LASIK."
FDA Moves to Speed Approval of Generic Drugs
Proposal Aims to End Repeated Patent Extensions.
The FDA has initiated action to allow the earlier introduction of lower-cost, generic versions of drugs. A new rule proposed by the federal agency would eliminate the current practice that allows drug manufacturers to repeatedly obtain 30-month patent extensions to block the approval of generic versions of drugs whose patents are expiring.
The FDA will accept public comments on the proposed rule for 60 days and then issue a final ruling.
"This proposed rule change would bring relief from the high prices that American consumers frequently pay for prescription drugs," said U.S. Health and Human Services Secretary Tommy G. Thompson. "This proposal would not only move generic drugs to market more quickly, but could save consumers approximately $3.5 billion every year."
The FDA approves generic versions of drugs whose original patents have expired if the generic's manufacturer can demonstrate that they are equivalent to the brand-name drugs. However, under a 1984 law, the original patent-holder can be granted multiple 30-month "stays" that keep a generic version off the market while the original patent-holder asserts a variety of new patent claims. These delaying tactics have enabled patent-holders to fend off low-cost alternatives to their drugs for years.
Under the new FDA proposal, the 30-month stays would be eliminated and patent-holders would have to assert their rights through traditional patent infringement lawsuits. In addition, the proposed rule would limit the types of patents that could be used by patent-holders to block generic drug approvals. In the past, drug manufacturers have used newly acquired patents on packaging and other relatively insignificant innovations to extend their exclusive rights to key drugs.
"This proposal provides a common sense balance between providing patent protection for brand-name pharmaceutical manufacturers and our desire to speed generic drug approval," said Lester Crawford, D.V.M., Ph.D, deputy commissioner of the FDA.
IN THE NEWS
CEO has no MBA. Bausch & Lomb Chairman and CEO Ronald L. Zarrella forfeited his 2002 annual bonus of at least $1.1 million, but kept his job, after taking full responsibility for falsely claiming he had an MBA degree. Zarrella attended NYU's Graduate School of Business at night for several years in the 1970s but didn't earn a degree. Initially, Zarrella had said that the reference to an MBA had gotten into his biography because he failed to proofread the biographical material. The misrepresentation was brought to light by financial columnist Herb Greenberg.
Visudyne benefits. Results from two extended Phase III clinical trials indicate that most patients who undergo Visudyne therapy for predominantly classic subfoveal wet AMD continue to show stable visual acuity at 36 and 48 months after initial treatment. About half of the patients in the 48-month follow-up study required a treatment in their fourth year to maintain their vision.
A sad note. Joe Alvarez, product specialist for the Heidelberg Retina Angiograph, died recently at the age of 51. Mr. Alvarez, who received ophthalmology training in the military and at Baylor College of Medicine, was a certified ophthalmic technician and a published lecturer.
Settlement. Allergan has settled patent infringement complaints involving its glaucoma medication Lumigan brought against the company by Pharmacia and Columbia University. Allergan will make an initial $120 million payment to Pharmacia and also pay royalties based on future sales of Lumigan. Pharmacia is the developer of the glaucoma medication Xalatan.
Symposium. The Contact Lens Association of Ophthalmologists will hold its annual meeting as part of the first Contact Lens and Eyecare Symposium, to be held in Lake Buena Vista, Fla., from Jan. 23 through 26.








