Focused on
New Goals
IOLs reflect a growing
emphasis on quality of vision, ultra-small incisions, light transmission and
more.
Introduction by Mark Packer, M.D.
Advances in IOL lens materials and design continue to create both opportunities and challenges for cataract and refractive surgeons. On the one hand, they herald improved biocompatibility and optical quality, reduction of capsular opacification, smaller incision size, restoration of accommodation and elimination of refractive error. On the other hand, new, more sophisticated lens designs demand more of us; we have to provide more accurate biometry, IOL power calculation and flawless surgical technique. Fortunately, advances in axial length measurement, software for IOL power calculation and phacoemulsification technology are allowing us to meet these higher standards.
Noteworthy advances in IOL technology include:
Biocompatibility. Biocompatibility means more than the absence of harmful effects; it means harmonious interaction between the IOL and the eye. The term "capsular biocompatibility," recently proposed by Michael Amon, essentially reflects maintenance of a clear capsule with minimal fibrosis and contraction.
Uveal biocompatibility (which usually refers to the absence of an inflammatory response and is quantified by the incidence of foreign-body giant cell reaction and aqueous flare) is a critical aspect of IOL material evaluation.
Reduction of PCO. Research by David Apple and Okahiro Nishi has demonstrated that both IOL design and surgical technique can help to reduce posterior capsular opacification. Careful surgeons create a capsulorhexis that completely overlies the optic; they also employ cortical cleaving hydrodissection and fastidious cortical clean up.
At the same time, squared posterior optic edge design and angulation of the haptics help to prevent capsular opacification. Many IOLs now feature these design elements.
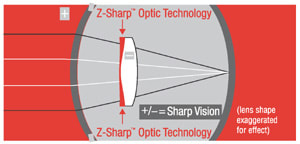
Optical quality. As refractive surgery moves toward the correction of higher-order aberrations, IOL technology is following. For example, Z-Sharp technology from Pharmacia corrects the spherical aberration of the cornea, improving pseudophakic contrast sensitivity to levels enjoyed by people in their youth. Also, Rolf Preussner has shown with his Okulix program that it's now possible to customize the design of an IOL to correct even large amounts of aberration, such as might occur after a decentered corneal ablation.
Meeting higher-order expectations
Cataract surgery and refractive surgery are merging as cataract patients come to expect excellent visual function without spectacle correction. At the same time, surgeons are beginning to offer refractive lens exchange to patients who want to decrease or eliminate their dependence on glasses or contact lenses. (We'll do well to remember that correcting lower-order aberrations such as sphere and cylinder form the foundation for the correction of higher-order aberrations.)
Improved tools are helping us meet these heightened standards:
- Accurate biometry, using immersion ultrasound or optical partial coherence interferometry, has been shown to improve the accuracy of IOL power calculation.
- When predicting spherical equivalents, using third-generation IOL power calculation formulas and outcomes analysis, such as the Holladay IOL consultant program, allows reduction of mean absolute error to theoretical limits.
- Using limbal relaxing incisions or toric IOLs to reduce pre-existing corneal astigmatism, or to compensate for surgically induced astigmatism, represents a critical step forward in successful refractive lens surgery.
- Cool bimanual phacoemulsification systems and IOLs such as the ThinOptx design will allow further reduction of incision size. This may eliminate surgically induced astigmatism.
- Forthcoming products such as the light-adjustable Calhoun Vision IOL, which allows noninvasive, post-implantation adjustment of residual refractive error, will help us keep the promise of emmetropia for pseudophakes.
Achieving the "Holy Grail"
Functional pseudophakic accommodation has now become an important goal. While the multifocal Array IOL has reduced spectacle dependence for many patients, unwanted optical side effects and loss of contrast sensitivity have hampered its implementation. However:
- A new foldable, diffractive bifocal IOL from Alcon that may offer an improvement is now under investigation.
- Axial movement designs, such as the C&C Vision CrystaLens, have shown good results in clinical trials without reducing contrast sensitivity. However, ongoing capsular contraction could pose a problem for long-term function.
- Other possibilities, such as refilling the capsule with a flexible injectable polymer, are still on the horizon of IOL development.
Pseudophakic accommodative technology still faces many challenges, including cataract extraction through a tiny capsulorhexis, intraoperative adjustment of refractive power and elimination of capsular opacification. But with technology advancing at its current pace, solutions to these problems may not be long in coming.
The end is not in sight
Advances in phacoemulsification and IOL technology have worked hand in hand to improve surgical results. However, we still have a long way to go before we can offer our patients the kind of "perfect vision" they'd like to have. Despite tremendous progress, as Howard Fine often reminds us, the story of intraocular lenses is still unfolding. j
Dr. Packer is a board certified ophthalmologist in private practice with Drs. Fine, Hoffman & Packer in Eugene, Ore., and clinical assistant professor of ophthalmology at Oregon Health & Science University in Portland. He's an active fellow with the American Academy of Ophthalmology, and a member of both the American Medical Association and the Oregon Medical Association.
Restoring Lost Contrast Sensitivity
The Tecnis Z9000 IOL from Pharmacia.
This fall, Pharmacia expects to submit to the FDA final clinical data on how its Tecnis Z9000 IOL with Z-Sharp optic technology affects contrast sensitivity in post-cataract patients.
|
|
|
|
The Tecnis Z9000, designed to improve contrast sensitivity in post-cataract patients, has all of the mechanical and material properties of the CeeOn Edge lens, but it's modified with a
prolate anterior surface. |
|
While current IOLs improve visual acuity, they don't address reduced contrast sensitivity, which can profoundly affect quality of life and safety for post-cataract-surgery patients and older people in general. Lead investigator Mark Packer, M.D., of Eugene, Ore., explained the thinking behind the Tecnis: "Wavefront technology has allowed us to determine why contrast sensitivity declines with age and after IOL implantation. The lens is the problem."
Dr. Packer reviewed research showing that a young, healthy lens, which has negative spherical aberration, compensates for whatever positive spherical aberration exists in the cornea. But, as the eye ages, the negative aberration in the lens steadily increases, eventually becoming positive at about age 40. This causes an overall positive spherical aberration, which reduces contrast sensitivity and functional vision.
Because today's IOLs have positive spherical aberration, the same phenomenon occurs after implantation. In fact, the patient's new net spherical aberration can be worse than it was preoperatively. The Tecnis, Dr. Packer explained, has an aspheric, prolate anterior surface and negative spherical aberration, which like a young, healthy lens should compensate for corneal aberrations and produce crisp vision.
At the American Society of Cataract and Refractive Surgery (ASCRS) symposium in June, Dr. Packer presented interim results from his inter-individual study of contrast sensitivity in patients implanted with the Tecnis compared with those implanted with a standard IOL (the acrylic, 6-mm AR40e Sensar). Patients were randomized to one of the two lenses following extraction of a visually significant cataract in one eye. The first implantation was performed in January; data on 15 eyes were available. Best-corrected mesopic and photopic contrast sensitivity was assessed post-op with sine wave (spatial frequency) grating tests.
Dr. Packer reported that Tecnis patients:
- demonstrated significantly better contrast sensitivity at 3, 6, 12 and 18 cycles per degree (cpd) spatial frequencies in photopic light conditions
- demonstrated significantly better contrast sensitivity at 1.5, 3 and 6 cpd in mesopic conditions
- experienced a 38% improvement in mesopic contrast sensitivity and a 41.8% improvement in photopic contrast sensitivity at the important peak region of 6 cpd.
No significant difference in contrast sensitivity was seen between the two lenses at 12 and 18 cpd under mesopic conditions.
The Tecnis interim clinical data is encouraging for cataract surgeons and patients, Dr. Packer said. "These data suggest that wavefront technology will allow us to address the problem of spherical aberration and contrast sensitivity inherent in current IOL technology and potentially bring important safety and quality of vision benefits to our post-cataract patients. Functional vision is the new frontier in cataract and refractive surgery."
In Search of the Smallest Incision
The ThinLens from ThinOptx.
In what he calls a major step for cataract surgery and for patients, Jorge Alio, M.D., Ph.D., medical director of Instituto Oftalmologico de Alicante in Spain, has been successfully implanting the ThinOptx ThinLens through incisions as small as 1.45 mm. "We are now looking to sub-1-mm-incisions, which means that we will be able to perform cataract surgery from beginning to end by a kind of puncture rather than an incision," he said. "We've been using the ThinOptx roller/injector, which allows us to perform a fast technique and decrease the risk of contamination from handling the lens."
The approximately 12 ThinLenses that Dr. Alio has implanted are stable, and in all cases patients achieved the best-corrected vision that was expected and are happy with the results of their surgeries.
"No special parasitic reflexes or flashes are visible in any of these lenses," Dr. Alio said. "I performed YAG laser capsulotomy in one case with an adequate result. Also, in one case of capsular instability, the lens was implanted at the ciliary sulcus without further problems."
According to ThinOptx, the experience of the other international surgeons working with the ThinLens is similar to Dr. Alio's. Approximately 100 lenses have been implanted, and:
- The posterior capsule opacification rate is lower than the industry standard. "The first group of 20 patients are out 10 months, and we only have one YAG to date," said Jim Simms, vice president for regulatory issues at ThinOptx. "We have some theoretical ideas about our ability to inhibit PCO, but this will take more time to prove via extended clinical study."
- Several investigators have tested for glare, and none has been noted. "The edges of ThinOptx lenses are very thin and therefore do not create glare," Simms said. "We now have bench testing that shows glare should be eliminated with our lens."
- No adverse effects have been reported with ThinOptx lenses. Also, Simms said, "A number of doctors are reporting that some of their patients are gaining both near and far vision with ThinOptx lenses."
|
|
|
|
Even with the use of the new roller/ injector, the ThinLens has been implanted through incisions as small as 1.45 mm. (Images courtesy of Matteo Piovella, M.D.) |
The ThinLens is made of a hydrophilic material; however, the company said it has the capability to make it from a hydrophobic acrylic material as well. The overall length of the lens is 11.2 mm; the optic is 5 mm. The haptic footplates are 50 microns thick from 10 mm to the tip of each haptic. The extreme thinness of the tip allows it to roll or curl, and the rolling creates little force on the capsular bag or the lens. Also, the ThinLens haptics have teardrop fenestration holes; when they're pointing clockwise, the surgeon knows that the continuous curved surface is resting against the posterior capsule.
Simms said the company is currently developing a version of the ThinLens that will allow implantation through an incision smaller than 1 mm. The first goal is to obtain the CE mark for the current version for sales in Europe. Work with the FDA is expected to begin by the end of this year.
Minimizing the Hazards of Blue Light
|
|
|
|
Five +20D lenses of each style were measured using a
spectrophotometer. The Acrysof Natural lens measured for this comparison is the commercial model, SN60AT (6.0 mm, 13.0-mm overall length, UV/blue light-absorbing
chromophore). The
investigational model mentioned in the accompanying text is the SB30AL (5.5 mm, 12.5-mm overall length, UV/blue light-absorbing chromophore). |
|
The Acrysof Natural from Alcon.
Alcon is awaiting final FDA approval of its single-piece Acrysof Natural IOL, which is designed to approximate the light transmission properties of the natural crystalline lens to absorb high-energy UV and blue light. A 0.04% yellow chromophore is covalently bonded to AcrySof Natural.
During the American Society of Cataract and Refractive Surgery (ASCRS) symposium in June, Stanley Chang, M.D., Columbia University, summarized research on blue light and the retina, concluding that "There is enough experimental and clinical evidence to suggest that blue light may present a hazard to the aging eye." The possibility of retinal damage is likely enhanced today, he said, because of the thinning ozone layer, increased lighting in our environment, increased use of mercury and xenon light, our aging population, and an increased volume of cataract surgery, much of which is also performed earlier in life. Dr. Chang also reviewed studies of AMD progression after cataract removal and IOL implantation, some of which show a correlation.
Paul Ernest, M.D., TLC Eye Care, Jackson, Mich., explained that some IOLs currently on the market absorb UV light, which ranges from 280 to 400 nm on the spectrum. However, they don't absorb the potentially damaging blue light, which makes up the 430 to 470 nm range; the AcrySof Natural does, he said. (See chart)
At the ASCRS meeting, Dr. Ernest presented results of the six-site clinical study assessing the safety and effectiveness of the AcrySof Natural (model SB30AL) compared with control model SA30AL. 300 patients underwent bilateral implantation (30 to 60 days apart) of either the AcrySof Natural or the SA30AL.
|
|
|
|
Studies have shown that the Acrysof Natural provides a UV and visible light transmission spectrum similar to the natural crystalline lens. |
Patients had to have bilateral age-related cataracts and pass the Farnsworth-Munsell D15 and Ishihara color vision tests. There were no statistically significant differences between the two study populations. Dr. Ernest said data from 120 to 180 days after the second eye implantation showed the AcrySof Natural to be comparable to the SA30AL on:
- best corrected visual acuity
- color perception (Farnsworth-Munsell D15)
- contrast sensitivity in mesopic and photopic light (VectorVision CSV-1000E test).
Patients filled out quality-of-life questionnaires, but results weren't available at the meeting. Dr. Ernest also said that tests on the Acrysof Natural demonstrated no cytotoxicity, mutagenicity or sensitivity and that the material and chromophore are stable.
Summing up his assessment of the Acrysof Natural, Dr. Ernest said, "There's really no downside."
The First FDA-Approved Accommodative IOL?
The AT-45 CrystaLens from C&C Vision.
It's quite possible that California-based C&C Vision's AT-45 CrystaLens will be the first accommodative IOL approved in the United States for implantation in cataract surgery patients. The reason: A key U.S. clinical trial is nearing completion, with data for 300 patients expected to be submitted to the FDA later this year. No other accommodative IOL is currently in clinical trials in the United States.
|
|
|
|
The AT-45 CrystaLens from C&C Vision
accommodates by vaulting forward when the ciliary body contracts. About 2,000 of the lenses have been implanted worldwide during the past 10 years. |
CrystaLens is already a proven product. It has the CE mark, with about 2,000 eyes implanted worldwide in the past decade.
Made of a proprietary silicone material, the CrystaLens functions by contraction of the ciliary body, which causes the lens to vault forward. Vision improves progressively after surgery and continues to improve throughout the postoperative course.
According to C&C Vision, most surgeons can implant the CrystaLens flat through a 3.5 to 4.0 mm incision. The company says one critical factor in obtaining good surgical results with the lens is obtaining an accurate axial length measurement, which can be achieved by doing immersion biometry prior to surgery. Surgeons who have used the CrystaLens also recommend manual keratometry to determine the curvature of the cornea.
Based on clinical trials, CrystaLens has achieved the following results at 1-year post-op:
For near vision:
- 77% can see J1 without glasses
- 91% can see J2
- 97% can see J3.
For distance vision:
- 93% can see 20/25 or better.
"The company is very optimistic about the future of CrystaLens," says J. Stuart Cumming, M.D., F.A.C.S., inventor of the CrystaLens and chief scientific officer for C&C Vision.
Coming Soon: An Acrylic Accommodative
The Akkommodative 1CU from Human Optics.
Human Optics AG of Erlangen, Germany, is making plans to begin U.S. clinical trials of its accommodative IOL, the Akkommodative 1CU, in 2003. The Akkommodative 1CU received the CE mark in June 2000 and has thus far been implanted in the eyes of more than 2,500 cataract surgery patients, primarily in Europe and Japan.
The Akkommodative is an acrylic lens that has four points of contact with the capsular bag; it uses the remaining powers of the ciliary muscle to move within the eye. Surgeons can implant the Akkommodative 1CU using the same techniques they would use to implant any other foldable lens.
|
|
|
|
The Akkommodative 1CU from Human Optics creates an
accommodative range of as much as 2.5D in 3 to 6 weeks. The lens has been implanted in more than 2,500 patients
worldwide. |
|
According to Human Optics, the IOL creates an accommodative power of up to 2.5D in 3 to 6 weeks, which allows patients to read newspaper print without any further visual correction.
"After 2 years of follow-up, we can state that the Akkommodative 1CU restores the optical and physical functions of the natural crystalline lens in most patients," says company spokesman Thomas Homscheid.
The company recommends that surgeons use the Zeiss IOLMaster for biometry prior to inserting the IOL. The IOLMaster measures axial length, keratometry and anterior chamber depth, and can be easily programmed to automatically calculate the power of the IOL for any patient.
Human Optics is currently establishing contacts with cataract surgeons in the United States who might be interested in participating in U.S. clinical trials for the Akkommodative 1CU.
"We want to make our innovative solutions accessible to U.S. eye surgeons as soon as possible," concludes Homscheid.
A New IOL Made of Collamer -- and a Smoother, More Consistent Injector
The Collamer Three-Piece IOL and Environmentally Controlled Cartridge from STAAR Surgical.
Two new products have just been introduced by STAAR Surgical:
The Collamer three-piece lens. This is the only three-piece IOL with a lens made from STAAR's 100% pure copolymer material, consisting of covalently bonded collagen and UV-absorbing chromophore. According to STAAR, this material offers numerous advantages over other lens materials:
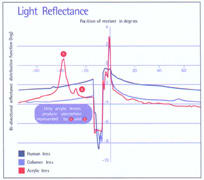
- Unlike acrylic lenses, Collamer's light transmitting and reflectivity characteristics are nearly identical to those of the human crystalline lens. This minimizes internal reflections, so patients don't experience halos or glare, or secondary images like those that can occur with acrylic lenses. (See chart, below.)
- Collamer is exceptionally biocompatible. The material carries a negative ionic charge that repels protein in the aqueous humor, and the lens attracts a monolayer of fibronectin once it's placed in the eye, preventing protein deposits and keeping the body's defenses from recognizing the lens as a foreign object. Also, the polymer is pure, with no residual monomers or viruses, bacteria or prions, which reduces the likelihood of low-grade inflammation, uveitis and iritis.
- Collamer repairs itself after exposure to YAG laser energy. The laser energy disburses the water molecules in the polyHEMA-collagen matrix, but within minutes they return to their original state. Surgeons report that within 15 to 20 minutes any pitting in the lens caused by the laser disappears.
- According to STAAR, Collamer is "exceptionally foldable," allowing minimal incision size. The lens emerges slowly and smoothly from the injector, and unfolds in the eye gently.
The Environmentally Controlled Cartridge. STAAR has also introduced a new IOL insertion cartridge that uses several new design advances to make delivering a lens into the eye smoother and more manageable.
The treated cartridge consistently maintains a high level of lubrication; it generates five times less friction than some other cartridges. When used with the STAAR plunger-type injector, this results in a noticeable increase in tactile control.
|
|
|
|
A comparison of light passing through three lens materials. Note that the Collamer data are very close to that of a natural human lens, while the acrylic material causes noticeable aberrations. |
Three layers of packaging protect the cartridge from environmental shifts commonly encountered during shipping and storage and in the operating room, ensuring that each one is in excellent condition when you open it. An outer foil (not sterile) is opened during prep. Inside is a sealed Tyvek pouch containing a sterile tray that holds the cartridge. This pouch is sealed during manufacture using a special process that preserves the moisture content.
According to STAAR, the cartridge can be loaded before surgery, but doesn't need to be advanced prematurely when used with STAAR's plunger-type injector.
Robert Bahr, M.D., currently chief of the division of ophthalmology at Miriam Hospital and an assistant clinical professor of surgery at Brown University, has inserted more than 2,000 plate IOLs made of Collamer during the past 2 years. "The only plate lens I've ever been totally happy with is STAAR's Collamer lens," he says. "The Collamer plate IOL performs as well as a 3-piece lens, in terms of inflammation, PCO and centration, which traditionally isn't true for plate lenses.
"The new three-piece design allows the Collamer material to be used in a more familiar framework. And it's well-designed; it's a modified C-loop, as opposed to a modified J-loop. The haptic is a little more flexible, with a softer curve that makes it easier to dial and position. I like the idea of flexible haptics that allow me to safely place the lens in the bag if the posterior capsule has a small opening, or in the sulcus, neither of which can be done with a plate lens.
"Because the three-piece is so new, I've only implanted a few of them, but I plan to use it more in the future."
Dr. Bahr is also happy with the new environmentally controlled cartridge. "The delivery is very nice. The new injector lets me implant a 3-piece lens with a full 6-mm optic through a 3-mm incision."
Squaring Off Against PCO
The Square-Round edge CV232SRE from CIBA Vision Surgical.
|
|
|
|
The CV232SRE IOL from CIBA Vision features a new squared posterior edge, with a rounded anterior edge to minimize glare. |
|
CIBA Vision Surgical reports that its popular CV232 IOL has now been redesigned with a squared edge on the posterior side to facilitate 360-degree capsular contact and help minimize PCO. Like its predecessor, the MemoryLens, the new CV232SRE also has a rounded front edge to minimize glare. (The lens material and haptics remain unchanged.)
Initial clinical results from European surgeons confirm that the lens makes 360° contact with the posterior capsule. So far, no lens epithelial cell proliferation has been observed, and no patient complaints of glare or other dysphotopsias have been reported.
Other advantages of the lens, according to CIBA Vision, include:
- high biocompatibility of the multi-polymer acrylic material, with low rates of bacterial adhesion and protein absorption
- no need for a folder or injector. The CV232SRE comes pre-rolled; it unfolds gradually and centers itself once you've placed it in the capsular bag. This minimizes risk to ocular tissue, saves time in the OR, and allows easy removal of viscoelastic.
Matteo Piovella, M.D., director of Centro Microchirurgia Ambulatoriale in Monza, Italy, has worked with this type of prerolled multipolymer acrylic IOL for more than a decade. He reports that the new lens maintains the best characteristics of the previous model. "This lens doesn't require folding, which saves time, and you can implant it in most eyes through a 3.2- to 3.5-mm incision. Also, the forceps used for insertion never enter the incision, so the phaco wound doesn't have to be enlarged during the procedure."
The CV232SRE has received marketing approval in both Europe and the United States. It's available in Europe and should be available in the United States later this year.
Good Grades for a Hybrid Edge Design
The Clariflex and Sensar IOLs with OptiEdge from Advanced Medical Optics (AMO).
|
|
|
|
AMO's OptiEdge design features a rounded anterior edge to scatter light and reduce internal reflections, a sloping side edge to direct
reflections away from the retina, and a squared posterior edge to facilitate 360° capsular
contact. |
Several doctors have reported new data regarding AMO's Clariflex foldable IOL (silicone) and Sensar foldable IOL (acrylic), both of which now feature the OptiEdge hybrid edge design. The OptiEdge combines three characteristics: a rounded anterior edge to scatter light and reduce internal reflections, a sloping side edge to reduce edge glare, and a squared posterior edge to facilitate 360 capsular contact. (This design was a response to the increase in internal reflections that resulted from squaring the posterior edges of IOLs to reduce PCO rates.)
The Clariflex lens with OptiEdge. Elizabeth Davis, M.D., who practices at Minnesota Eye Consultants, P.A., in Bloomington, Minn., and is assistant clinical professor at the University of Minnesota, shared the results of a recent study she and her partner, Thomas Samuelson, M.D., conducted between May 2001 and April 2002. They followed 146 eyes of patients ranging in age from 35 to 95 years that they implanted with the Clariflex lens. Implant powers ranged from 12D to 26D.
Drs. Davis and Samuelson report that they found:
- no intraoperative complications
- no post-op complications
- no IOL decentration
- no prolonged or recurrent inflammation
- no posterior synechiae
- no cystoid macular edema (CME)
- no IOL-related vision complaints (glare, halos, etc.).
- In terms of post-op gain in lines of BCVA: At 1 to 3 months patients had gained an average of 2.62 ± 2.09 lines (with a range of 0 to 10 lines).
The doctors noted that they haven't observed any PCO among these patients yet, but they'll need to wait a year before drawing any conclusions.
Regarding her personal experience using the Clariflex lens, Dr. Davis says, "I've found the Clariflex easy to fold, load and inject. It unfolds in the eye in a very controlled, predictable way and has excellent, secure centration in the capsular bag. Sulcus fixation is an equally viable alternative." She also notes that using the new Clariflex requires no change in technique or wound size compared with the previous generation lens (the SI40NB).
The Sensar lens with OptiEdge. Steven Dewey, M.D., who practices in Colorado Springs, Colo., is very satisfied with the new design of the Sensar. "The Sensar with OptiEdge has become my primary implanted lens," he says. "The edge design is fascinating. The squared posterior edge induces a capsular bend which makes the posterior capsule clearer over time, and the slightly angled middle edge and rounded anterior edge have eliminated symptomatic dysphotopsias."
Dr. Dewey recently performed a retrospective review of 50 patients to see whether a new modified version of AMO's Sapphire Small Incision Inserter would make a significant difference in the size of the incision when implanting the Sensar with OptiEdge. He inserted the Sensar through an unenlarged 2.8-mm phaco wound, and consistently measured the post-insertion incision at 3.0 to 3.1 mm. In contrast, with the original Sapphire inserter, the post-insertion incision was 3.4 mm, and with forceps, he had to first enlarge the incision with a 3.5-mm slit blade to allow for the final 3.8-mm internal wound size.
Proving It's Got What It Takes
An update on Hydroview from Bausch & Lomb.
Introduced in the United States last November, the Hydroview is the first hydrophilic acrylic IOL from Bausch & Lomb. It's also the first one-piece foldable lens manufactured in the United States. The Hydroview is available in two sizes -- the H60M, a 6-mm lens for larger pupils and eyes, and the H55S, a 5.5-mm IOL.
Hydroview has been designed to offer the advantages of a foldable small-incision implant, with the stability and centration of a one-piece rigid lens, according to Bret L. Fisher, M.D., of Panama City, Fla., who served as a primary investigator for Hydroview's U.S. clinical trial.
|
|
|
|
The Hydroview acrylic IOL from Bausch & Lomb continues to draw positive reviews from surgeons and patients. More than 600,000 have now been implanted worldwide. |
|
"I'd feel comfortable using this lens in any of my cataract patients," says Dr. Fisher. "My personal experience with the lens is that all patients who have no co-existing ocular pathology could expect to achieve excellent corrected distance and near acuity with these lenses, equal to or exceeding what I've come to expect with other modern lens implants. Because it's highly biocompatible, I especially like it for patients with a history of uveitis, or in combined cataract/glaucoma procedures. The biocompatibility of the lens lessens postoperative inflammation and speeds healing."
Dr. Fisher says the Hydroview unfolds in a controlled fashion and can be implanted easily by any surgeon familiar with using foldable lens implants; only standard keratometry and biometry measurements are required prior to surgery.
Douglas Grayson, M.D., of New York City, has implanted about 1,500 Hydroview IOLs. He also praises the biocompatibility of this IOL and the "excellent" visual acuity it offers patients.
"The Hydroview cuts more easily than silicone or pure acrylic IOLs if it needs to be removed in an IOL exchange procedure," notes Dr. Grayson. "This factor becomes more of a consideration with the advent of post-LASIK patients needing cataract surgery, since IOL calculations can be notoriously unpredictable."
"The Hydroview has been well received in the United States, judging from the comments made to me by my colleagues at various meetings I've attended," concludes Dr. Fisher. "This is an IOL that's been used internationally for a number of years, with more than 600,000 implants to date worldwide. So it's safe to say that this is a product whose performance has been proven."