Practice Watch
Tips and News You Can Use
FDA Approves "Custom-CAP" Method to Correct Poor LASIK Outcomes
VISX Treatment Fixes Off-Center Ablations.
|

|
|
|
With the Custom-CAP Method, the laser beam is split into an array of seven beams, which are then directed to the area of the highest corneal elevation. |
|
A recent FDA ruling has given new hope to individuals who underwent a laser vision correction procedure that resulted in debilitating visual problems.
In late February, the FDA approved the Custom-Contoured Ablation Pattern ("Custom-CAP") Method developed by VISX Inc. Approval was granted under a Humanitarian Use Device exemption. Such exemptions are given for medical devices that can benefit fewer than 4,000 individuals per year.
The Custom-CAP Method is a treatment system used to remove microscopic amounts of tissue from the cornea to correct off-center ablations resulting from prior laser vision correction surgery. The treatment reshapes the cornea for optimum correction. Off-center ablations can produce such serious visual defects as glare, double vision and halos.
With Custom-CAP treatment, ophthalmologists can use a VISX Star S3 ActiveTrak excimer laser, together with a Humphrey Atlas corneal topographer and associated VisionPro Ablation Planning Software to reshape the cornea through a topographically driven treatment that's precisely controlled by size, depth and location. According to VISX, the Custom-CAP Method can correct off-center ablations resulting from a previous treatment with any brand of laser.
Patients with abnormally thin corneas and those exhibiting signs of keratoconus shouldn't undergo Custom-CAP treatment.
"This indication will permit physicians to assist those individuals who were previously untreatable with conventional laser ablation methods," says Steven Trokel, M.D. "Treating these patients is a priority for VISX and all refractive surgeons."
You can receive Custom-CAP treatment certification by having your site approved by an institutional review board and attending a VISX training seminar offered at the annual meeting of the American Society of Cataract and Refractive Surgery in Philadelphia in June.
Zeiss Diagnostic Device Receives FDA Approval
Optical Coherence Tomography Helps Detect Retinal Problems and Glaucoma.
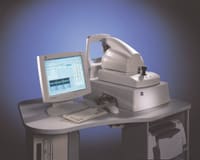
Carl Zeiss Ophthalmic Systems Inc. of Dublin, Calif., has received FDA clearance to market its Optical Coherence Tomographer Model 3000, known as the OCT3, a diagnostic device capable of providing in vivo cross-sectional imaging of the nerve fiber layer, the macula and the optic nerve head.
The company says the OCT3, which sells for approximately $50,000, is the first product to employ optical coherence tomography technology in a diagnostic imaging device that can be used by practitioners to conduct thorough eye examinations for retinal diseases and glaucoma. The technology had previously been used only in research applications.
Optical coherence tomography works like ultrasound, but uses light rather than sound to achieve better resolution, according to the company. The OCT3 provides real-time, cross-sectional images of retinal tissue that have axial resolution of 10 microns or less.
"This technology dramatically improves the physician's ability to characterize the retinal layer during routine eye examinations," says Jim Taylor, president and CEO of Carl Zeiss Ophthalmic Systems. "The OCT3 makes a rigorous retinal screening possible in minutes without discomfort. We also see great potential for future clinical applications as clinical usage increases."
In typical examinations, physicians depend on a visual examination of the surface of the retina to reveal signs of disease inside. The OCT3 enables physicians to bypass invasive procedures and see below the surface of the retina to directly measure internal retinal structures as an aid in the diagnosis of glaucoma and retinal diseases. A typical OCT3 exam requires less than 10 minutes.
Optical coherence tomography was invented at the Massachusetts Institute of Technology in 1991. Carl Zeiss Ophthalmic Systems purchased exclusive rights from MIT to develop and market the technique for ophthalmic diagnostic use. The inventors of optical coherence technology -- James Fujimoto, Eric Swanson and Carmen Puliafito, M.D. -- have been awarded the 2002 Rank Prize for opto-electronics. This prestigious prize is given for achievements that are considered to be of significant benefit to mankind.
Government Wants Better Monitoring of ASCs
OIG Report Says Medicare System Has been Lax.

The explosive growth of ambulatory surgery centers (ASCs) in the past decade has outpaced the Medicare system's ability to monitor and regulate quality standards at these facilities.
That's the conclusion reached in a report recently issued by the Office of the Inspector General (OIG) of U.S. Department of Health and Human Services. And while the report says that state certification agencies and independent accreditors could be doing more follow-up checks to verify that ASCs are meeting their standards on an ongoing basis, the OIG singles out the Centers for Medicare & Medicaid Services (CMS) for not holding the state agencies and accreditors to higher standards of accountability.
The OIG also wants CMS to institute a formal consumer complaint process and make more information about the performance of individual ASCs available to the public. CMS has overall quality oversight responsibility for the more than 3,000 ASCs that perform procedures covered by Medicare.
The Federated Ambulatory Surgery Association (FASA), which represents the interests of ASCs throughout the country, said it welcomes the OIG's efforts to review CMS oversight, but suggests that the report does a "disservice" by not also questioning Medicare's oversight of hospital outpatient departments and physicians' offices.
FASA President Bob Williams expressed concern that "Medicare beneficiaries and other patients will be misled to believe that surgery in an ASC is unsafe. Nothing could be further from the truth."
The Outpatient Ophthalmic Surgery Society (OOSS) said in a statement that the OIG's findings "appear to be appropriately focused and balanced, though sharply critical of CMS and the state agencies that conduct surveys for the Medicare program."
The OIG report emphasizes that oversight of ASCs is now more important than ever because of the dramatic growth in the number of procedures performed in these facilites. About 12,000 procedures were performed in ASCs in 1990. In 2000, ASCs accounted for more than 100,000 procedures.
According to the OIG report, CMS has until now been largely content to allow state certification agencies and independent accreditors -- such as the Accreditation Association for Ambulatory Health Care and the Joint Commission on Accreditation of Healthcare Organizations -- to determine whether ASCs are meeting compliance and quality-of-care standards. However, the report notes that a third of ASCs certified by state agencies haven't been recertified in 5 or more years. Accredited ASCs are surveyed at least every 3 years, but the report faults the survey process for not devoting enough attention to verifying compliance.
To correct these deficiencies, the OIG wants CMS to play a more active role in the ASC oversight system: by getting more directly involved in the monitoring process and by making state certification agencies and accreditors more accountable for their own performance in overseeing these facilities.
REFRACTIVE SURGERY UPDATE
Lactoferrin test. CIBA Vision and Touch Scientific Inc. are offering the TouchTear MicroAssay System, a quick, pre-LASIK test that measures lactoferrin concentration in the tear film. Low levels of preoperative lactoferrin have been shown to correlate with LASIK undercorrections and high levels of the protein have correlated with overcorrections. By knowing a patient's preoperative lactoferrin level, a surgeon can use punctum plugs prior to LASIK to achieve a more normal measurement at the time of the procedure.
Sunrise cutback. Sunrise Technologies, which manufactures the LTK Hyperion laser for the treatment of hyperopia, has dismissed nearly all of its employees due to financial losses.
Surgeon survey. Results from a recent survey of 1,511 members of the American Society of Cataract and Refractive Surgery indicate that 19% of the respondents said they had refractive surgery on their own eyes. This group reported performing significantly more refractive surgery than those eye surgeons who
hadn't had a refractive procedure.
Procedure pickup. TLC Laser Eye Centers Inc. said it performed more than 28,400 paid laser vision correction procedures in the 3 months ending Feb. 28. That represents a 40% increase from the 17,700 procedures performed in the previous quarter, but is still well below the 33,500 procedures performed in the same period a year ago.
Acquisition. Paradigm Medical Industries, Inc. has purchased Innovative Optics of Albuquerque, N.M., which manufactures and markets microkeratomes and related equipment used in microkeratome procedures. Innovative Optics, which had sales of less than $1 million last year, produces the Innovatome microkeratome.
IN THE NEWS
|

|
|
|
The Nidek DC-3300 System |
|
Laser approved. The Nidek DC-3300 Diode Laser System has been approved by the FDA for use in all retinal photocoagulation procedures, including limited and pan-retinal photocoagulation, transpupillary laser photocoagulation, transcleral photocoagulation and endophotocoagulation. The DC-3300, which has a list price of $35,000, can also be used for treatment of diabetic retinopathy and macular degeneration, as well as certain glaucoma procedures, including laser trabeculoplasty and iridotomy. The laser system is designed to operate in combination with various delivery systems, such as slit lamps, binocular indirect opthalmoscopes and endoprobes.
Alcon IPO. Nestle has completed an initial public offering of 25% of Alcon, its wholly owned eyecare business. The $2.3 billion offering, consisting of 69.7 million shares of Alcon common stock, began trading on the New York Stock Exchange on March 21 at a price of $33. Alcon's stock symbol is ACL.
Suit dropped. Bausch & Lomb has voluntarily dropped its preliminary injunction motion to prevent CIBA Vision from selling its Focus Night & Day 30-day continuous-wear contact lenses. Bausch & Lomb had initially filed suit in November, claiming that CIBA Vision had infringed on the silicone hydrogel technology B&L uses to manufacture its PureVision contact lenses. The PureVision lenses were also recently approved for 30 days and nights of continuous wear.
Acquisition. Ocular Sciences, a leading maker of contact lenses, has acquired certain assets of its Japanese distributor, Seiko Contact Lens, for approximately $21 million. Ocular expects the transaction to boost earnings in fiscal 2003.
Envision development. Bausch & Lomb said pre-market development and production of its Envision TD drug-delivery system will take place at its Waterford, Ireland, facility. The Envision TD system, currently in clinical trials for posterior uveitis, diabetic macular edema and AMD, is essentially a tiny implant that's infused with medicine and inserted into the back of the eye. The medicine is then released in a steady, sustained-released flow directly inside the eye for up to 3 years.








