Clinical News: Medical and Product Updates
DIABETIC MACULAR EDEMA
Envision TD
Recently, Bausch & Lomb presented results of a clinical study of its Envision TD technology for the treatment of diabetic macular edema (DME). As you know, DME is a complication of diabetes in which chronic deterioration and leakage of blood vessels in the retina cause progressive loss of sight.
Treatment using Envision TD technology involves a surgeon placing an implant into the back of the eye. The Envision TD implant delivers fluocinolone acetonide to the diseased area of the eye for up to 3 years. Fluocinolone acetonide is an anti-inflammatory steroid compound that controls inflammation and inhibits the growth of damaged blood vessels.
Five patients with DME participated in the clinical study. The patients, who hadn't responded to laser treatment, each received the Envision TD implant in one eye; the other eye wasn't treated (the control group). All five patients had resolution of their macular edema in the treated eye, as confirmed by fluorescein angiography. The mean visual acuity in the five treated eyes improved from 20/158 to 20/82 at 9 months. The visual acuity in the control group of eyes remained essentially unchanged.
The patients tolerated the implant, although they did experience a rise in intraocular pressure (IOP). (This is an expected side effect of fluocinolone acetonide.) Researchers treated the IOP effectively with eye drops.
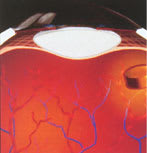
The FDA has granted "fast-track"
status for approving the Envision TD.
AMD
Miniaturized Telescope
A new device may improve visual acuity and quality of life for your patients who suffer from age-related macular degeneration (AMD).
The device, called the Implantable Miniaturized Telescope (IMT), is manufactured by VisionCare Ophthalmic Technologies Inc.
Researchers implant the 3.0X telescope in the posterior chamber, in place of the eye's natural lens; haptic loops hold the IMT in position. The company reports that the surgical procedure is similar to that for implanting standard intraocular lenses. Researchers only implant the IMT in one eye to provide central vision, while the non-operated eye provides peripheral vision. The IMT contains a number of microlenses that magnify objects in the patient's central visual field to improve central vision in the implanted eye without external low-vision aids.
At press time, the company had enrolled 10 eligible participants in the trial. The company needs five more participants before the trial can commence. To enroll in the trial, patients must:
- be at least 60 years old
- have visual acuity as similar as possible in both eyes
- have no major eye disease or problem except for cataracts
- have bilateral stable AMD consisting of an atrophic or disciform scar
- have visual acuity not better than 20/80 and not worse than 20/400 in either eye.
For more information, call the company at (408) 872-9394, or visit www.visioncare.co.il.

Patients require at least 1 month
to adapt to the IMT
CATARACTS
Antipsychotic drugs
As you know, studies have suggested that lens changes and ocular disturbances sometimes occur in persons taking antipsychotic drugs. But a recent study found that general antipsychotic drug use isn't associated with the occurrence of cataracts.
In the study, researchers estimated the incidence of cataracts in schizophrenic patients and compared it with the incidence in the general population. Researchers also examined the role of antipsychotic drug use on the risk of cataract development.
Results showed that, overall, antipsychotic drug users weren't at a greater than average risk of developing cataracts. However, patients who use prochlorperazine or chlorpromazine may have a greater than average risk.
The researchers concluded that schizophrenia itself isn't associated with an increased risk of developing cataracts.
Epidemiology 2000; 11: 620-3.
AMD
Lutein
Here's good news for your patients who are at risk for AMD. A study shows that supplements of natural lutein esters increase macular pigment density. Mac-ular pigment is thought to protect against AMD.
In the study, eight male volunteers took lutein ester supplements derived from marigolds, providing a daily dose of 10 mg of lutein for 12 weeks. All subjects showed an increase in plasma lutein concentration.
Am. J. Ophthalmol. 2000; 129: 832

Lutein may reduce the risk of AMD.
GLAUCOMA
IOP and visual damage
An Advanced Glaucoma Interven-tion Study (AGIS) has associated low intraocular pressure (IOP) with reduced progression of visual field defects during 6 or more years of follow-up.
Investigators used two types of analysis. In the first analysis (predictive) they categorized 738 eyes into three groups based on IOP determinations during the first three 6-month follow-up visits. Eyes with early average IOPs greater than 17.5 mm Hg worsened more than eyes with average IOPs less than 14 mm Hg. By the third visit the difference was approximately one unit of visual field defect score (VFDS). The difference between the groups was even greater by the 7-year follow-up.
In the second analysis (associative) investigators categorized 586 eyes into four groups based on the percent of 6-month visits (during the first follow-up years) in which eyes presented with an IOP less than 18 mm Hg.
Eyes with IOPs less than 18 mm Hg during the 6-year period had almost no mean changes from baseline in VFDS. Conversely, eyes with IOPs greater than 18 mm Hg in more than half of the follow-up visits had an estimated worsening of 0.63 units of VFDS. The difference was even greater at 7 years.
Am. J. Ophthalmol. 2000; 130: 429-440.
CLINICAL DRUG TRIALS
Alliance
In hopes of regaining some lost ground, Harvard University Medical School has formed an alliance with four other medical schools to secure more clinical drug trials. The alliance -- the Multicenter Academic Clinical Re-search Organization (MACRO) -- feels that private industry is taking over too much of the human research on new medical treatments. The schools are promising drug companies faster approval and completion of critical clinical trials.
Typically, large private institutional review boards approve clinical trials within a week, while academic medical centers require 4 to 8 weeks. MACRO has tried to reduce approval time to 2 to 4 weeks, but doesn't anticipate 1-week turnarounds.
"I find that unsettling," said a MACRO spokesperson. "It's almost like a rubber stamp."
A decade ago, academic medical centers conducted 90% of the clinical trials paid for by drug companies, compared to 40% today. Harvard wins less than 1% of the $20 billion to $25 billion that drug companies award in research grants each year.
An official at Harvard said, "Trials are getting done more often in small storefront doctors' offices and small community hospitals and solo practice settings. There's less bureaucracy, but there's also less intellectual scientific oversight and input."
DIABETIC RETINOPATHY
After phaco
Recently, researchers set out to determine two things: the rate of progression of diabetic retinopathy following phacoemulsification, and whether surgeon experience and/or surgical duration adversely affect visual outcome.
Researchers reviewed 150 eyes of 119 diabetic patients who underwent phaco over 5 years.
Results of the study showed that visual acuity improved by two or more lines in 78% of the eyes; 62% of the eyes had a final visual acuity of at least 20/40. Researchers saw retinopathy progression in 25% of the eyes with 6 to 10 months of follow-up. Retinopathy progression was statistically associated with preoperative nonproliferative diabetic retinopathy, prolonged surgical duration and limited surgical experience.
The study authors concluded that the visual results and rate of retinopathy progression after phaco didn't appear to differ significantly from those reported using other techniques. However, nonproliferative diabetic retinopathy, longer surgical duration and surgical inexperience resulted in an increased rate of retinopathy progression.
Study Indicates Ophthalmologists Can Play a Key Role in Preventing the Onset of MS
According to researchers involved in the CHAMPS study, ophthalmologists can play a crucial role in detecting the earliest signs of multiple sclerosis (MS) and delaying the onset of the disease. Information on CHAMPS (Controlled High-Risk Subjects Avonex MS Prevention Study) was presented as part of the "Hot Topics" session at the American Academy of Ophthalmology meeting this past October.
Researchers reported that treatment with Biogen Inc.'s Avonex (interferon beta-1a) significantly reduces the rate at which high-risk individuals develop clinically definite multiple sclerosis (CDMS).
On the lookout for optic neuritis
The first indication that a person may have MS is a single, clinical, demyelinating event of the optic nerve, spinal cord, or cerebellum/brain stem. High-risk individuals often first experience damage to the optic nerve, which presents as blurry or lost vision. This places ophthalmologists at the front lines in terms of identifying an early sign of MS.
"Nearly 50 percent of individuals who are eventually diagnosed with MS first present with optic neuritis. By then, these individuals already have active MS in the brain," CHAMPS researcher Steven Galetta, M.D., Director, Neuro-Ophthalmology Service and Van Meter Professor of Neurology/Ophthalmology at the University of Pennsylvania Medical Center, told ophthalmologists at the AAO session. "Therefore, it is essential that ophthalmologists recognize optic neuritis as a potential harbinger of MS, even at the very first presentation, and refer at-risk patients for an MRI. These high-risk patients should be identified and treated early with Avonex because there is now evidence that we can delay the onset of the disease and slow its progression."
An MRI can identify patients who are at high-risk for developing MS by revealing clinically silent spots on the brain. A positive MRI indicates a very high chance of a second attack within 2 to 3 years, if left untreated.
"There can be as many as 10 to 12 flare-ups in the brain for each clinical symptom of MS, including optic neuritis," said Lawrence Jacobs, M.D., principal investigator for the CHAMPS study. "MS smolders in the brain, causing permanent damage between the first and second attack."
About the CHAMPS study
The CHAMPS study was a randomized, double blind, placebo-controlled, Phase III clinical trial involving 383 patients determined to have a high probability of developing CDMS based on brain MRI changes and clinical events consistent with MS. Patients received weekly intramuscular injections of either 30 mcg of Avonex or placebo. The study was conducted at 50 clinical centers in the United States and Canada. Findings included:
- The rate of development of CDMS was 44% lower in the Avonex-treated group than in the placebo-treated group.
- The increase in brain MRI T2 lesion volume was 91% lower in the Avonex-treated group than in the placebo-treated group.
- With the results of this study showing a strong benefit of Avonex treatment at the time of the first clinical event, there is added justification for obtaining brain MRIs at the earliest symptoms of MS. Previously, MRIs were considered useful in predicting the possibility of developing CDMS but were not considered necessary because they wouldn't affect treatment and patient management.
Helping to shape standard of care
"To date, there are no accepted guidelines for treating patients who have experienced a single MS-like attack but who have not yet developed clinically definite MS," Dr. Galetta said. "This study is extremely important because it indicates that initiating therapy with Avonex in high-risk patients at the first sign of optic neuritis, or other neurological symptoms, with silent MRI lesions can significantly delay the development of the disease."
Jennifer Goodwin, contributing editor








