PRACTICE
WATCH
Tips And New You Can Use
2002 Fee Schedule Holds Bad News for Physicians
Across-the-Board 5.4% Cuts are in Store if the Conversion Formula Isn't Changed.
After winning several battles that resulted in increased Medicare reimbursement for specific procedures, ophthalmologists are now waging the biggest reimbursement battle of all.
Unless Congress responds quickly to the complaints of physician organizations and changes the conversion formula that's used to determine Medicare payments to providers, all physicians will experience an across-the-board 5.4% cut in Medicare reimbursement beginning Jan. 1.
Both the American Academy of Ophthalmology (AAO) and the American Society of Cataract and Refractive Surgery (ASCRS) used strong language to criticize the method by which the conversion formula is calculated.
An ASCRS statement calls the formula "flawed" and notes that annual adjustments in Medicare payments are tied to the performance of the Gross Domestic Product (GDP).
"Declining GDP growth will generally lead to physician payment cuts," says the statement. "Though the GDP has no impact on healthcare needs, an economic downturn can lead to steep reductions in Medicare payments to physicians."
The AAO says that despite intense lobbying efforts by the "entire physician community," the Centers for Medicare and Medicaid Services (formerly HCFA) has "steadfastly refused to correct the errors in the formula."
A bill was recently introduced in the Senate to change the conversion formula, but since the events of Sept. 11, Congress has focused on terrorism and attempts to boost the economy.
Both the AAO and ASCRS are concerned that Congress may not get around to re-evaluating the formula until 2002.
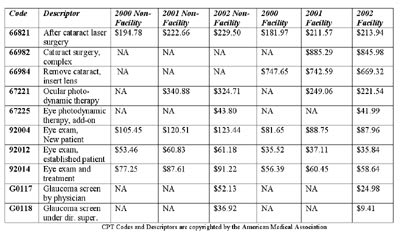
Medicare payments for selected CPT codes: 2000, 2001 and preliminary 2002 fees.
IN THE NEWS
Referral network. A spokesperson for the mail-order contact lens distributor 1-800-Contacts says the company may start its own network of eyecare providers to whom it will refer patients. The company says such a network "is in the cards" as a response to contact lens manufacturers who refuse to sell product to 1-800-Contacts and providers who won't release contact lens prescriptions to patients.
Generic latanoprost. Par Pharmaceuticals has filed an abbreviated new drug application (ANDA) seeking FDA approval to manufacture and market latanoprost ophthalmic solution 0.005%, the generic equivalent of Pharmacia's leading glaucoma medication, Xalatan. Par's ANDA states that existing Xalatan patents are either invalid, unenforceable, or won't be infringed by the Par product. A Pharmacia spokesperson says his company has enforceable patents for Xalatan and will vigorously contest any attempts to market the generic product.
Macular edema. Oculex Pharmaceuticals, Inc., which develops drug delivery systems for the eye, is enrolling patients suffering from persistent macular edema in Phase II trials of its Posurdez delivery system. Posurdex is a biodegradable, micro-sized delivery system that's placed inside the eye to provide continuous drug therapy to a targeted site.
New leadership. Bausch & Lomb has named Ronald Zarrella chairman and CEO and a member of its board of directors. Zarrella returns to Bausch & Lomb after 7 years with General Motors, most recently serving as president of the North American division. Until 1994, Zarrella held senior management positions with B&L and was a director of the company. Zarrella follows William Waltrip, who had been acting as B&L's interim chairman and CEO.
Glaucoma treatment. Presby Corp. says its Scleral Expansion Band procedure, which is currently in clinical trials as a treatment for presbyopia, has also demonstrated effectiveness in reducing intraocular pressure in glaucoma patients. The company cited a study involving 24 patients over a 9-month period.
Acquisition complete. CIBA Vision has completed its integration of Wesley Jessen's systems and is now operating as a single company worldwide.
Appointed. Randall S. Baldwin has joined Gerber Coburn Optical Inc. as a group product manager, overseeing all product management activities associated with the Gemini Fine-to-Coat Process, Step One blocking systems, and Stratum and LTI coating systems.
Xalatan sales. Pharmacia Corp. said worldwide sales of its Xalatan glaucoma medication rose to $221 million in the 3 months ending Sept.30, an increase of 19% over the same year-ago period.
Licensing agreement. Bausch & Lomb has licensed the exclusive worldwide rights to a patented multifocal soft contact lens design from Unilens Corp., USA. Bausch & Lomb will develop, manufacture and market a cast-molded, frequent replacement multifocal soft contact lens using the Unilens technology.
Visudyne sales. QLT Inc., the co-developer of Visudyne photodynamic therapy for wet AMD, reported Visudyne sales of $57.5 million for the 3-month period ending Sept. 30, an 86% increase over the same period a year ago.
Market share. Allergan now has an 18.6% share of the United States glaucoma medication market, an increase of 4.4% since Jan. 1. The company launched two new glaucoma medications this year, Lumigan and Alphagan P.
NovaMed exits. NovaMed Eyecare is discontinuing its management services operations and will now primarily focus on its surgical facilities business. NovaMed operates 15 single-specialty ambulatory surgical centers and 15 laser vision correction centers.
Italian business. Bausch & Lomb has acquired Fidia Farmaceutici's Italian ophthalmic pharmaceuticals business, Fidia Oftal, based in Catania, Italy. Fidia Oftal's product line includes over-the-counter and prescription products, including dry eye preparations, anti-infectives, ointments, viscoelastics and nutraceuticals.
Acquisition. Vision Pharmaceuticals, which markets Viva-Drops for dry eye through ophthalmologists, optometrists and retail outlets, has been acquired by Corneal Sciences Corp., a privately owned pharmaceuticals company.
New CEO. Ocular Sciences, a leading maker of soft contact lenses, has named Stephen J. Fanning president and chief executive officer. Fanning previously served as president of Johnson & Johnson's McNeil Specialty Products division. Fanning succeeds John Fruth, continues as Ocular's chairman.
Companies Battle Over Contact Lens Technology
At Issue is the Patent for the Silicone Hydrogel Material.

According to independent studies, silicone hydrogel lenses can supply far more oxygen to the eye than ordinary disposable lenses and 40% more oxygen than the minimum threshold recognized for overnight wear. The oxygen-permeable nature of silicone hydrogel allows the lenses to be worn for longer periods without causing discomfort to the wearer.
In early November, Bausch & Lomb filed suit against CIBA Vision in the U.S. District Court for the Western District of New York for infringing on its patent 6,312,706, relating to extended wear soft contact lenses. The patent was issued the day before Bausch & Lomb filed suit, but the company said the timing of the patent award had no bearing on the company's decision to file suit. B&L is seeking an injunction to prevent CIBA Vision from marketing Focus Night & Day contact lenses.

"Bausch & Lomb was the first to invent the silicone hydrogel technology, the first to manufacture a commercial lens using this technology, and the first on the market with a highly oxygen-permeable extended wear soft lens," said Mark M. Sieczkarek, Bausch & Lomb senior vice president and president of the Americas Region.
Steve Osbaldeston, president of CIBA Vision's North American Lens Business Unit, noted that CIBA Vision sued B&L in 1999 for infringing on four patents relating to Focus Night & Day technology and, after a re-examination process, all four patents were reissued to CIBA Vision last year.
"This Bausch & Lomb action is an attempt to create a bargaining chip to gain access to CIBA Vision's valuable patents," concluded Osbaldeston.
For more information on the continuous wear option, see "A New Era in Continuous Wear."
Marketing Musts
Three Ways to Market Smarter, Not Harder.
Brad Ruden, president of MedPro Consulting and Marketing Services, says marketing a medical practice isn't just advertising -- it encompasses the entire patient experience.
The key is to market smarter, not harder, he says. The best marketing practices aren't necessarily the ones who spend the most, but those who follow a few fairly simple rules and master them well. Here are three ways to market more effectively:
Don't stagnate. A medical practice is a business and needs to be evaluated and re-evaluated to continually improve. One- and three-year strategic plans, as well as comprehensive year-end reviews, will help you to identify areas for improvement.
Analyze existing patients. Your new patients will likely be very similar to your current patients -- choosing you for the same reasons. The more you can learn about why patients choose you, the more effective your marketing will be.
Be where your patients are. Select the advertising or promotional opportunities that reach the greatest portion of your target audience -- not just those that reach the greatest number of people in general. One survey indicated that a family's healthcare providers are primarily chosen by the wife or mother of the household. Is your advertising accessible to these decision-makers in content and placement?
Look for more tips next month.
REFRACTIVE SURGERY UPDATE
Presbyopia treatments. SurgiLight says a study of 100 patients who underwent a scleral ablation procedure using the company's OptiVision YAG laser system at four overseas sites achieved excellent results in reversing prebyopia. After 1 year, 82% of those treated were able to read with no need for correction. SurgiLight has requested FDA approval to begin a major clinical study in the United States. Another treatment for presbyopia, Presby Corp's Scleral Expansion Band Procedure, has received FDA approval for expanded clinical trials.
InterWave. Emory Vision, an independent vision correction practice affiliated with the Emory University School of Medicine in Atlanta, is using its new InterWave vision and eye measurement technology to more accurately predict how a patient will see after having LASIK. InterWave takes up to 70 different measurements of each eye, employing interactive technology that allows patients to input real-time information on what they're seeing during the eye exam. InterWave is being used as a diagnostic tool, both with new patients and as an aid in helping to improve the vision of patients who had their original surgery elsewhere
Imaging system. Nidek Inc. has submitted a 510(k) application to the FDA for evaluation of the image restoration function of the Nidek Advanced Vision Information System (NAVIS). The company says the image restoration function enables eyecare professionals to enhance captured images for greater detail, exposing images that wouldn't otherwise be observed and providing better diagnoses.
CEO resigns. Thomas E. Wilson has resigned as CEO of laser vision correction provider LCA-Vision, effective Dec. 31. Company chairman Stephen N. Joffe will act as interim CEO.








